PHYTANE
Synonym(s):2,6,10,14-Tetramethylhexadecane
- CAS NO.:638-36-8
- Empirical Formula: C20H42
- Molecular Weight: 282.55
- MDL number: MFCD00060946
- EINECS: 211-332-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
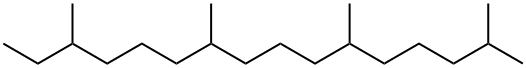
What is PHYTANE?
Description
Phytane is a 20–carbon atom branched-chain alkane. Formally known as 2,6,10,14-tetramethylhexadecane, it has three chiral centers and therefore eight possible stereoisomers. It is normally found as a mixture of most if not all of its isomers.
Unlike lower alkanes that are flammable and present breathing hazards, phytane is essentially nonhazardous. As a chemical, it has no practical uses or commercial value.
Why is phytane a Molecule of the Week? It turns out to be quite useful to geoscientists as a marker of carbon history and, in particular, as a proxy for long-ago carbon dioxide levels in Earth’s atmosphere.
Phytane is a degradation product of chlorophyll, which, of course, is related to CO2 consumption. Jaap Sinninghe Damsté and her co-workers at the Royal Netherlands Institute for Sea Research obtained more than 300 ocean sediments and oils from different locations and geological periods, extracted phytane from them, and determined the 12C/13C ratios in the phytane.
Photosynthesis, and therefore chlorophyll, consumes 12CO2 preferentially to 13CO2. So when the atmosphere is rich in CO2, phytane from chlorophyll has a high 12C/13C ratio. Thus, the researchers could estimate atmospheric CO2 back to the Cambrian period, ≈500 million years ago. Their results matched well with those from existing CO2 proxies that covered shorter time periods.
Chlorophyll is known to have existed as long ago as 1.7 billion years. Geoscientists therefore have the challenge of using proxies to determine atmospheric CO2 levels much farther back than the Cambrian.
The Uses of PHYTANE
Phytane is a useful intermediate, which is involved in the manufacturing and analysis of various compounds. Phytane has recently been used in the development of a stable and environmental-friendly lubricating oil. By determining the ration of !3C to 12C in phytane isolated from ocean sediments researchers in the Netherlands have been able to quantify CO2 levels reaching back to the Cambrian period when animals emerged on the earth.
Definition
ChEBI: Phytane is a diterpene.
Properties of PHYTANE
| Boiling point: | 69-71 °C0.001 mm Hg(lit.) |
| Density | 0.791 g/mL at 20 °C(lit.) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Hexane (Slightly) |
| form | Liquid |
| appearance | colorless liquid |
| color | Clear Colorless |
| BRN | 1744639 |
| EPA Substance Registry System | Phytane (638-36-8) |
Safety information for PHYTANE
Computed Descriptors for PHYTANE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1