Phosphatidylserine
- CAS NO.:51446-62-9
- Empirical Formula: C42H82NO10P
- Molecular Weight: 792.07
- MDL number: MFCD00135635
- EINECS: 000-000-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
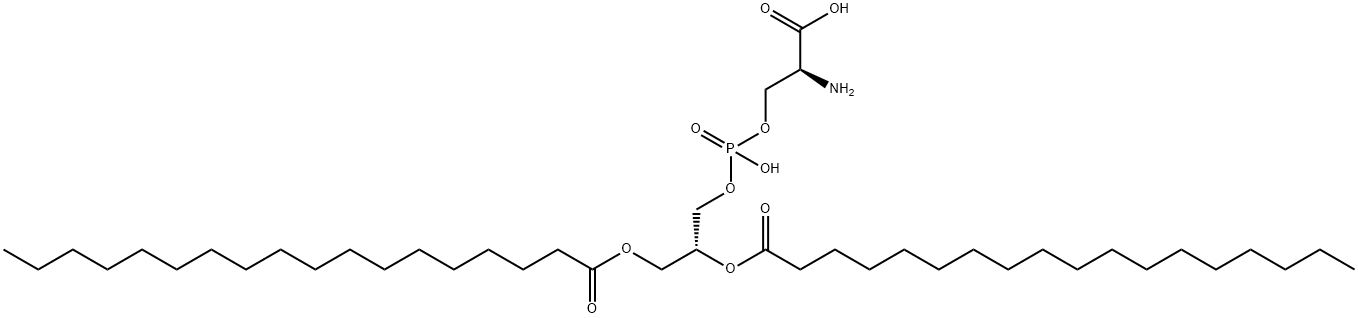
What is Phosphatidylserine?
Description
Phosphatidylserine (PS) is quite literally a “brain nutrient.” As a matter of fact, this phospholipid is an integral component in the structure of the brain and spinal cord, and is active at cell membranes (including synaptic membrane zones).It plays a key role in cell cycle signaling, specifically in relationship to apoptosis. Studies have provided evidence that PS may promote attention, memory, processing speed, and support overall cognitive health. PS may also support healthy stress and cortisol levels. A significant amount of published clinical research has demonstrated that PS supplementation supports various cognitive parameters in children, including those with ADHD.
The Uses of Phosphatidylserine
Phosphatidylserine is a brain-boosting supplement. It is a class of phospholipids found in cell membranes. Its levels and location within the brain can affect important signaling pathways for cell survival and communication. Phosphatidylserine includes two fatty acids that can vary from saturated or monounsaturated to polyunsaturated omega-6 and omega-3 versions like docosahexaenoic acid (DHA). Some clinical trials of phosphatidylserine supplements have shown modestly improved cognitive function, but better designed trials reported no benefit.
Definition
ChEBI: 1,2-distearoyl-sn-glycero-3-phosphoserine is a 3-sn-phosphatidyl L-serine in which the phosphatidyl acyl group at both positions 1 and 2 is stearoyl. It has a role as a mouse metabolite. It is functionally related to an octadecanoic acid.
Biosynthesis
In mammalian cells, PS is endogenously synthesized via two pathways, both of which exchange the head group of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) for serine, generating PS in both instances (Vance, 2008). PS synthase-1 (PSS1) catalyzes the choline exchange reaction and PS synthase-2 (PSS2) catalyzes the ethanolamine exchange reaction.
PS Synthase 1: Phosphatidylcholine + L-Serine--->Phosphatidylserine + Choline
PS Synthase 2: Phosphatidylethanolamine + L-Serine--->Phosphatidylserine + Ethanolamine
PS, both from endogenous synthesis and exogenous supplementation can be hydrolyzed via two pathways. This occurs via phospholipases, which are located in the plasmalemma (Leventis & Grinstein, 2010). The sn-1acyl chain of PS is hydrolyzed via the action of PSspecific phospholipase A1, while the sn-2 acyl chain of PS is hydrolyzed via PS-specific phospholipase A2, with lysophosphatidylserine (LPS) being formed in both cases. Lysophosphatidylserine produced through the hydrolysis of the sn-2 acyl chain has been implicated in a number of biological processes including mast cell activation and neural differentiation (Hossono, Aoki, Nagai, Bandoh and Ishida (2001); Aoki, Nagai, Hosono, Inoue, and Arai (2002).
stars.library.ucf.edu
Benefits
Phosphatidylserine is a phospholipid found in the membranes of brain cells and has a protective effect on the cells.Phosphatidylserine plays an important role in keeping the mind and memory sharp as a nutritional supplement. It also helps in relieving stress, sleep and Alzheimer's disease.
Biological Functions
Phosphatidylserine (PS) is a glycerophospholipid consisting of a phosphatidyl group attached to L-serine via a phosphodiester linkage. PS is a critical component of the cellular plasma membrane and accounts for 2-15% of plasma membrane lipid composition, depending on the cell or tissue type. The highest concentrations of PS are found in neuronal tissues, which are critical for maintaining conduction velocity in myelinated neurons, as well as for higher order cognitive skills such as learning and memory. In normal, healthy cells, PS is held in the inner membrane surface (facing the cytosol) by the lipid transporter protein flippase. However, in apoptotic cells, PS molecules ‘shuffle’ between the inner and outer plasma membrane monolayers. When PS molecules flip to the extracellular (outer) surface of the cell membrane, they act as a signal for macrophages to engulf and digest the (apoptotic) cell.
Side Effects
When taken by mouth: Phosphatidylcholine is POSSIBLY SAFE when taken by mouth, in a dose up to 30 grams per day for 6 weeks, or up to 6 grams per day for 2 years. When phosphatidylcholine is taken by mouth, it can sometimes cause excessive sweating, stomach upset, and diarrhea.
When given as a shot: Phosphatidylcholine is POSSIBLY SAFE when given as an injection under the skin for up to 5 doses spread 2-4 weeks apart. The injections can cause irritation, swelling, redness, itching, burning, bruising, and pain at the injection site. These side effects usually go away over a period of several days. Sometimes, phosphatidylcholine might cause gastrointestinal upset, like bloating, diarrhea, and nausea.
If phosphatidylcholine is injected directly into a non-cancerous fatty tumor (lipoma), it might cause a reaction that could make the tumor more fibrous, needing surgery to remove it.
When applied to the skin: Phosphatidylcholine is POSSIBLY SAFE when applied to the skin for up to 12 weeks.
www.webmd.com
Safety
Small clinical trials suggest that phosphatidylserine supplements produce no serious adverse effects for elderly patients, although it may reduce blood pressure or increase body weight. Since some supplements are prepared from cow brains, it is theoretically possible for those supplements to transmit prion diseases such as mad cow, though no cases have been reported.
Source
Food sources of phosphatidylcholine include:
Egg yolks (6,771 mg/100g)
Pig liver (1,668 mg/100g)
Chicken liver (1,120 mg/100g)
Soybeans (917 mg/100g)
Squid (777 mg/100g)
Chicken breast (391 mg/100g)
Beef (408 mg/100g)
Peanuts (270 mg/100g)
Cod (331 mg/100g)
Spinach (37 mg/100g)
Potato (38 mg/100g)
Carrot (23 mg/100g)
Apple (21 mg/100g)
Cow's milk (12 mg/100g)
Properties of Phosphatidylserine
| Boiling point: | 816.3±75.0 °C(Predicted) |
| Density | 1.039±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| pka | 1.31±0.50(Predicted) |
| form | Liquid |
| color | Yellow |
Safety information for Phosphatidylserine
Computed Descriptors for Phosphatidylserine
| InChIKey | TZCPCKNHXULUIY-RGULYWFUSA-N |
| SMILES | C(O)(=O)[C@@H](N)COP(=O)(O)OC[C@H](OC(=O)CCCCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCCCC |
Phosphatidylserine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
