PHORBOL
- CAS NO.:17673-25-5
- Empirical Formula: C20H28O6
- Molecular Weight: 364.43
- MDL number: MFCD00065450
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
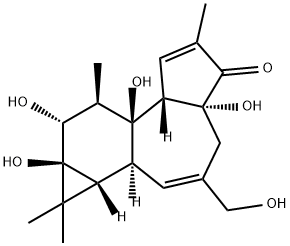
What is PHORBOL?
Description
Phorbol is a diterpene originally isolated from croton oil. It is used as a starting material for the semisynthesis of various phorbol diesters, which are structurally analogous to diacylglycerol and activate PKC isoforms by associating with their C1 domains.
Description
(+)-Phorbol is a long-known, structurally complex natural product. In 1934, Swiss chemists Bonifaz Flaschentr?ger and Rudolf von Wolffersdorff isolated it from hydrolyzed croton oil, which was a known tumor-promoting substance. Research teams led by E. Hecker in Germany and George Ferguson in Britain reported its structure in 1967.
According to the?Merck Index, “Phorbol has a structural skeleton based on cyclopropabenzazulene.” It also has a daunting eight stereogenic centers. Three research groups have published total syntheses of phorbol, but the first two produced the racemic compound. Just this year, Phil S. Baran and colleagues at the Scripps Research Institute (La Jolla, CA) and LEO Pharma (Ballerup, Denmark) found a way?to make phorbol enantioselectively?in 19 steps, less than half the number required in earlier work.
Baran’s group’s work and future, more efficient syntheses should provide sufficient quantities of phorbol and its esters for potential drug development. In addition to their ability to promote rapid tumor growth, phorbol derivatives may be useful immunotherapy, antiviral, and anticancer drugs.
The Uses of PHORBOL
Phorbol is a plant-derived diterpene that exhibits various biological activities such as its role as tumor promoters through the activation of protein kinase C.
What are the applications of Application
Phorbol is a plant-derived diterpene that is a tumor promoter
Definition
ChEBI: A diterpenoid with the structure of tigliane hydroxylated at C-4, -9, -12(beta), -13 and -20, with an oxo group at C-3 and unsaturation at the 1- and 6-positions.
Preparation
Phorbol can be extracted from croton oil by two-phase alcoholysis and purified by high-speed counter-current chromatography[1]. The specific method is as follows:
The optimized operation conditions of biphasic alcoholysis were a reaction time of 91 min, a temperature of 14°C, and a croton oil-methanol ratio of 1:30 (g:ml). The phorbol during the biphasic alcoholysis was 3.2-fold higher in content than that obtained in conventional monophasic alcoholysis. The optimized high-speed countercurrent chromatography method was using the ethyl acetate/n-butyl alcohol/water at 4.7:0.3:5 (v:v:v) with Na2SO4 at 0.36 g/10 ml as the solvent system, using the mobile phase flow rate of 2 ml/min, the revolution of 800 r/min, under which the retention of the stationary phase was achieved at 72.83%. The crystallized phorbol following high-speed countercurrent chromatography was obtained as high purity of 94%.
General Description
White solid.
Air & Water Reactions
Water soluble.
Reactivity Profile
PHORBOL is unstable to prolonged exposure to air, light and ambient temperatures. PHORBOL is also sensitive to acid and alkalis and is subject to autooxidation. PHORBOL dissolves slowly, therefore solution in a desired solvent is best accomplished by prolonged shaking under an inert atmosphere. .
Fire Hazard
PHORBOL is probably combustible.
Toxicology
Toxicity of Phorbol: Dermal (mouse) LD50: 36 mg - mild, Unlike its diesters, phorbol does not appear to be a co-carcinogen or to enhance chemical mutagenesis. Evidence of carcinogenicity is limited. Highly toxic by inhalation, in contact with skin and if swallowed. Irritating to eyes, respiratory system and skin.
References
[1] JIE-PING FAN. Biphasic alcoholysis coupled with high-speed countercurrent chromatography for high performance on separating phorbol from Croton tiglium Linn extracts[J]. Journal of separation science, 2023. DOI:10.1002/jssc.202200984.
Properties of PHORBOL
| Melting point: | 162-163° and 233-234°; mp 249-250° |
| Boiling point: | 415.62°C (rough estimate) |
| alpha | D24 +102° (water); D20 +118° (c = 0.4 in dioxane) |
| Density | 1.4 |
| refractive index | 1.4450 (estimate) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; Ethanol: slightly soluble; PBS (pH 7.2): 1 mg/ml |
| Water Solubility | Soluble in water |
| pka | 11.36±0.70(Predicted) |
| form | powder to crystal |
| color | White to Orange to Green |
| CAS DataBase Reference | 17673-25-5 |
| EPA Substance Registry System | Phorbol (17673-25-5) |
Safety information for PHORBOL
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for PHORBOL
| InChIKey | QGVLYPPODPLXMB-UBTYZVCOSA-N |
| SMILES | C1(=O)[C@]2(O)[C@]([H])([C@@]3(O)[C@H](C)[C@@H](O)[C@@]4(O)C(C)(C)[C@@]4([H])[C@]3([H])C=C(CO)C2)C=C1C |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 Phorbol CAS 17673-25-5View Details
Phorbol CAS 17673-25-5View Details
17673-25-5 -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
