Phloretin
Synonym(s):β-(4-Hydroxyphenyl)-2,4,6-trihydroxypropiophenone;2?,4?,6?-Trihydroxy-3- p-hydroxyphenylpropiophenone;2?,4?,6?-Trihydroxy-3-p-hydroxyphenylpropiophenone;2′,4′,6′-Trihydroxy-3-(4-hydroxyphenyl)propiophenone;3-(4-Hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)-1-propanone
- CAS NO.:60-82-2
- Empirical Formula: C15H14O5
- Molecular Weight: 274.27
- MDL number: MFCD00002288
- EINECS: 200-488-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-17 08:48:38
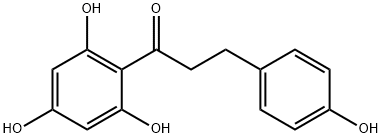
What is Phloretin?
Chemical properties
Crystalline Solid
Chemical properties
White to pale yellow solid.
The Uses of Phloretin
Phloretin has been used:
- to study its effect on the 2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose (2-NBDG) and 2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-l-glucose (2-NBDLG) uptake
- to incubate microvesicles in order to inhibit GLUT1 (glucose transporter 1)-mediated transport in radioactive ligand up-take assay
- as a component of KRH buffer to stop glucose uptake by trophoblast cells in vitro
The Uses of Phloretin
As glucose transport inhibitor, Phloretin can inhibits protein kinase C and has been shown to inhibit the entry of five enveloped viruses into human fibroblasts.
What are the applications of Application
Phloretin is a calcium channel protein inhibitor
Definition
ChEBI: Phloretin is a member of the class of dihydrochalcones that is dihydrochalcone substituted by hydroxy groups at positions 4, 2', 4' and 6'. It has a role as a plant metabolite and an antineoplastic agent. It is functionally related to a dihydrochalcone.
General Description
Phloretin is a dihydrochalcone flavonoid with antioxidant activity usually found in apples and apple-derived products. It is extensively used for peroxynitrite scavenging and the inhibition of lipid peroxidation. It has been found to inhibit the growth of several cancer cells and induce apoptosis of B16 melanoma and HL60 human leukemia cells.
Biological Activity
phloretin is a dihydrochalcone, a type of natural phenols which can be found in apple tree leaves and manchurian apricot. phloretin inhibits the active transport of glucose into cells via sodium-glucose linked transporter (sglt) 1 and 2 with ic50 value of 49±12 μm [2].sglts are a family of glucose transporter which is found in the small intestine mucosa (sglt 1) and nephron proximal tubule (sglt 2). they contribute to the renal glucose reabsorption.after treatment of phloretin, differentiated 3t3-l1 cells exhibited significantly enhanced glycerol and the inhibition of adipogenesis-related transcription factor that were regulated by sglts. additionally, phloretin promoted phosphorylation of amp-activated protein kinase and increased activity of adipose triglyceride lipase and hormone-sensitive lipase [1]. when raw 263.7 cells were cultured from differentiated 3t3-l1 cell media, pt suppressed the sglt-associated nuclear transcription factor kappab and mitogen-activated protein kinase pathways [1].in streptozotocin-induced rat model of diabetes type i, oral administration of phloridzin (5/10/20/40 mg/kg/day) resulted in significant reduction of blood glucose levels and improved dyslipidemia. additionally, administration of phloridzin reduced urine volume and water intake in a dose-dependent manner [3].
Biochem/physiol Actions
Reacts with vic-dicarbonyl compounds such as glyoxal and methylglyoxal, preventing cytotoxic conjugation with biological macromolecules.
Purification Methods
Crystallise phloretin from aqueous EtOH. [Zemplen & Bognár Chem Ber 75 1040 1942, Zemplén Chem Ber 76 386 1943, Beilstein 8 IV 3518.]
References
[1] huang w c et al. , phloretin and phlorizin promote lipolysis and inhibit inflammation in mouse 3t3-l1 cells and in macrophage-adipocyte co-cultures. mol nutr food res. 2013, 57: 1807-1817.
[2] kasahara t, kasahara m. expression of the rat glut1 glucose transporter in the yeast saccharomyces cerevisiae. biochem j. 1996, 315 ( pt 1):177-182.
[3] najafian m, jahromi m z, nowroznejhad m j, phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. mol biol rep. 2012, 39(5): 5299-306.
Properties of Phloretin
| Melting point: | ~260 °C |
| Boiling point: | 337.26°C (rough estimate) |
| Density | 1.1827 (rough estimate) |
| refractive index | 1.573-1.575 |
| FEMA | 4390 | 3-(4-HYDROXYPHENYL)-1-(2,4,6-TRIHYDROXYPHENYL)PROPAN-1-ONE |
| storage temp. | 2-8°C |
| solubility | Acetonitrile (Slightly), Methanol (Slightly) |
| pka | 7.16±0.40(Predicted) |
| form | Powder |
| color | White to beige |
| Odor | odorless |
| Water Solubility | soluble |
| Merck | 14,7326 |
| JECFA Number | 2022 |
| BRN | 1887240 |
| CAS DataBase Reference | 60-82-2(CAS DataBase Reference) |
Safety information for Phloretin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Phloretin
| InChIKey | VGEREEWJJVICBM-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Phloretin CAS 60-82-2View Details
Phloretin CAS 60-82-2View Details
60-82-2 -
 Phloretin 98% CAS 60-82-2View Details
Phloretin 98% CAS 60-82-2View Details
60-82-2 -
 Phloretin 99% (HPLC) CAS 60-82-2View Details
Phloretin 99% (HPLC) CAS 60-82-2View Details
60-82-2 -
 Phloretin, 99% CAS 60-82-2View Details
Phloretin, 99% CAS 60-82-2View Details
60-82-2 -
 Phloretin, 98% CAS 60-82-2View Details
Phloretin, 98% CAS 60-82-2View Details
60-82-2 -
 Phloretin CAS 60-82-2View Details
Phloretin CAS 60-82-2View Details
60-82-2 -
 Phloretin CAS 60-82-2View Details
Phloretin CAS 60-82-2View Details
60-82-2 -
 Phloretin CAS 60-82-2View Details
Phloretin CAS 60-82-2View Details
60-82-2