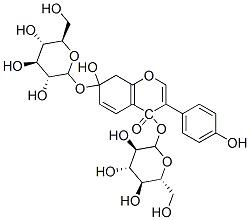
Puerarin
Synonym(s):8-(β-D -Glucopyranosyl-7-hydroxy-3- (4-hydroxyphenyl)-4H-1-benzopyran-4-one;Kakonein
- CAS NO.:3681-99-0
- Empirical Formula: C21H20O9
- Molecular Weight: 416.38
- MDL number: MFCD00063399
- EINECS: 609-296-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-25 18:47:31
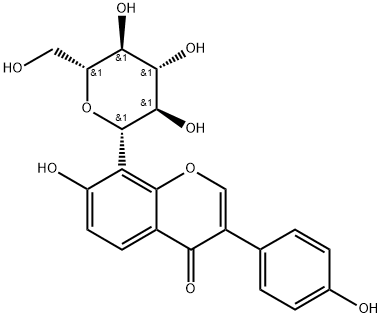
What is Puerarin?
Description
Puerarin, also known as pueraria flavonoids, is a kind of flavonoid glycoside
extracted from the roots of Pueraria alba or Pueraria thomsonii and is also one of
the main effective ingredients of Pueraria lobata. That Pueraria lobate was used to
treat diseases has already been recorded in China’s ancient medical books such as
Shen Nong’s Materia Medica, Treatise on Miscellaneous Diseases and Medical
Dictionary.
Kanzu root is widely distributed in our country and of rich resources. It
has been reported that puerarin could be extracted from Pueraria lobata (Wild.)
Ohwi, Radix Pueraria thomsonii, Pueraria omeiensiswanget Tang, Pueraria edulis
Pamp and Pueraria phaseoloides, but the content of puerarin differs.
Pueraria has a great value for nutrition and medicine and was considered the
south ginseng of China. Puerarin has been widespread concerned over our country
for its use of food and medicine in recent years.
Chemical properties
White powder
Physical properties
Appearance: White to light-yellow crystalline powder. Solubility: Soluble in methanol, freely soluble in ethanol, slightly soluble in water, insoluble in chloroform and ether. Melting point: 187–189?°C.
History
In 1959, Japanese chemist Shibata Shoji first studied the chemical production of
pueraria root, indicating that isoflavones are the main active ingredients of pueraria
root, including puerarin, daidzin and daidzein . Then in 2003, David Lee first
total synthesized analogical puerarin . And it was in 1974 that puerarin was first
extracted by Fang Qicheng and other chemists in China . Due to its strong activity of anti-cardiovascular and anti-cerebrovascular ischemia and hypoxia, expanding the coronary artery and cerebrovascular, reducing myocardial oxygen
consumption, and improving myocardial systolic and microcirculation function,
puerarin was approved for clinical use by the Ministry of Health in 1993.
In recent years,
researchers have synthesized a series of puerarin derivatives, and active research
showed that the part of the puerarin derivatives has a good biological activity and
bioavailability. In a new dosage form
research, researchers have developed several types of dosage forms such as quickrelease solid dispersions, solid from microemulsion preparation, etc., which greatly
increase the solubility of puerarin, thus improving bioavailability. In recent years,the research on crystal drug has opened up new ways for the application of
puerarin.
The Uses of Puerarin
Puerarin is a naturally occuring isoflavonoid derived from Chinese medical herb kudzu root and has been used for the treatment of various cardiovascular diseases. Recent sutdies have shown the potenti al of puerarin treatment as a novel approach to lowering the risk of or improving function in ischemia-reperfusion brain injury-related disorders.
The Uses of Puerarin
beta-adrenergic blocker
The Uses of Puerarin
Puerarin is a natural isoflavone isolated from plants of the genus Pueraria used in traditional Chinese herbal medicine. It is biotransformed by intestinal bacteria to give the phytoestrogens daidzein and equol , resulting in antithrombotic, antiallergic, and other salutary effects. When given intraperitoneally, puerarin evokes diverse responses by modulating serotonin receptors. This compound also suppresses lipopolysaccharide-mediated activation of NF-κB in RAW 264.6 macrophages when given at 20-40 μM.
What are the applications of Application
Puerarin is a flavonoid derivative with potential antioxidant activity
Definition
ChEBI: A hydroxyisoflavone that is isoflavone substituted by hydroxy groups at positions 7 and 4' and a beta-D-glucopyranosyl residue at position 8 via a C-glycosidic linkage.
Indications
At present the most widely used medicinal formulation of puerarin in clinical application is puerarin injection, with more than 90 enterprises producing it. The injection was mainly used for the treatment of coronary heart disease, angina pectoris, myocardial infarction, retinal movement, vein occlusion, sudden deafness and other diseases. Another dosage form of puerarin is eye drops for the treatment of primary open-angle glaucoma, ocular hypertension, primary angle closure glaucoma and secondary glaucoma. In addition, there are puerarin tablets and puerarin capsules, belonging to the health-care product.
Biochem/physiol Actions
Isoflavone component of kudzu. Reduces anxiety symptoms associated with alcohol withdrawal.
Pharmacology
Contemporary pharmacological research has demonstrated that puerarin has wide
pharmacological activities on the cardiovascular system, nervous system, liver
impairments, osteoporosis and hangover .
The clinical and experimental researches have shown that puerarin has a significant role in the prevention of cerebral vascular disease, which can expand the
coronary artery and improve the metabolism of an ischemic heart muscle, improving brain circulation, increasing cerebral blood flow and reducing blood volume and
heart rate. There is good control of puerarin on myocardial ischemia and cerebral
ischemic disease.
A large number of experiments have proved that puerarin can promote the glucose and lipid metabolism and reduce blood sugar and blood lipid. The protection
of puerarin on diabetes was attributed to its functions on inhibiting apoptosis and
oxidative stress.
Puerarin have protection efficacy on liver damage induced by chemical substances, alcohol, surgery and other experimental damage. This effect is closely
related to the antioxidant effect, resisting lipid peroxidation, inhibiting platelet
aggregation and improving circulation.
Puerarin can also improve the eye microcirculation obviously and reduce the
pressure of the eyes, thus acting obvious therapeutic effect on glaucoma. In addition, it has a variety of preparations, such as liquors, which are still under
development.
Clinical Use
Currently, puerarin is used clinically for the treatment of hypertension, coronary
heart disease, angina pectoris, arrhythmia, myocardial infarction, ischemic cerebrovascular disease, retinal arteriovenous obstruction, sudden deafness, diabetes complications, dizziness and other diseases. Besides, it also is used to treat chronic
pharyngitis, cerebral infarction and Parkinson’s syndrome.
However, puerarin also has adverse reactions in clinical therapy. Fever is the
main symptom. In addition, allergic dermatitis, anaphylactic shock, laryngeal
oedema, increased transaminase, gastrointestinal bleeding, haemolysis phenomenon and kidney damage occurred occasionally, and these symptoms disappeared
after withdrawal of the medication.
Properties of Puerarin
| Melting point: | 187-189°C |
| Boiling point: | 688.0±55.0 °C(Predicted) |
| Density | 1.614±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Powder |
| pka | 6.46±0.20(Predicted) |
| color | White to Off-white |
| Water Solubility | Soluble in DMSO or DMF. Slightly soluble in water or ethanol |
| λmax | 303nm(MeOH)(lit.) |
| BRN | 64198 |
| CAS DataBase Reference | 3681-99-0(CAS DataBase Reference) |
Safety information for Puerarin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Puerarin
| InChIKey | HKEAFJYKMMKDOR-NCHCSYJDSA-N |
| SMILES | OC1=CC=C2C(C(C3C=CC(O)=CC=3)=COC2=C1[C@H]1[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O1)O)=O |&1:18,19,20,22,24,r| |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran



You may like
-
 Puerarin CAS 3681-99-0View Details
Puerarin CAS 3681-99-0View Details
3681-99-0 -
 Puerarin 98% CAS 3681-99-0View Details
Puerarin 98% CAS 3681-99-0View Details
3681-99-0 -
 Puerarin CAS 3681-99-0View Details
Puerarin CAS 3681-99-0View Details
3681-99-0 -
 Puerarin CAS 3681-99-0View Details
Puerarin CAS 3681-99-0View Details
3681-99-0 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
