Phenyl chloroformate
Synonym(s):Chloroformic acid phenyl ester;Chloroformic acid phenyl ester, Phenyl chlorocarbonate;Phenyl chloroformate
- CAS NO.:1885-14-9
- Empirical Formula: C7H5ClO2
- Molecular Weight: 156.57
- MDL number: MFCD00000637
- EINECS: 217-547-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
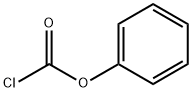
What is Phenyl chloroformate?
Chemical properties
clear oily liquid. Corrosive. Insoluble in water, soluble in ethanol, ether, easily soluble in petroleum ether.
The Uses of Phenyl chloroformate
Phenyl chloroformate is used in the synthesis of poly(2-(phenoxycarbonyloxy)ethyl methacrylate) and phenyl-(4-vinylphenyl) carbonate. It acts as a precursor of phenyl mixed anhydrides which are used in peptide coupling reactions. It serves as a dehydrating reagent for the conversion of primary amides to nitriles and an intermediate in the synthesis of pharmaceuticals and carbamates.
Preparation
Phenyl chloroformate is synthesized by the reaction of phenol with phosgene. Phenol was dissolved in chloroform, phosgene was introduced under cooling, and the absorbed phosgene was in an equal molar ratio to phenol, and equimolar N,N-dimethylaniline was added dropwise under stirring at 5-10 °C. Then add cold water to dilute, separate the oil layer, wash with dilute hydrochloric acid and water successively. After drying with anhydrous calcium chloride, chloroform is evaporated, and then distilled under reduced pressure to collect 74-75°C (1.73kPa) fraction, which is phenyl chloroformate. The yield is about 90%.
What are the applications of Application
Phenyl chloroformate is an important organic synthesis intermediate, which is widely used in chemical synthesis and can be used as polymer catalyst, plastic modifier, fiber treatment agent, and intermediate of medicine and pesticide.
General Description
Phenyl chloroformate appears as a colorless liquid with a strong odor. Toxic by ingestion, inhalation and skin absorption. Very irritating to skin and eyes. Used as a reagent for organic synthesis.
Air & Water Reactions
Emits fumes containing HCl in moist air. Decomposes in water to form HCl.
Reactivity Profile
Phenyl chloroformate is incompatible with strong oxidizing agents, alcohols, amines, alkali. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
Poison by inhalation. Moderately toxic by ingestion and skin contact. A corrosive sktn and eye irritant. See also ESTERS. When heated to decomposition it emits toxic fumes of Cl-.
Properties of Phenyl chloroformate
| Melting point: | -28 °C |
| Boiling point: | 74-75 °C/13 mmHg (lit.) |
| Density | 1.248 g/mL at 25 °C (lit.) |
| vapor density | 1 (vs air) |
| vapor pressure | 1.22 psi ( 20 °C) |
| refractive index | n |
| Flash point: | 168 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with N,N-dimethylformamide. |
| form | Liquid |
| color | Clear |
| Water Solubility | hydrolysis |
| Sensitive | Moisture Sensitive |
| BRN | 606778 |
| CAS DataBase Reference | 1885-14-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Carbonochloridic acid, phenyl ester(1885-14-9) |
| EPA Substance Registry System | Carbonochloridic acid, phenyl ester (1885-14-9) |
Safety information for Phenyl chloroformate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H290:Corrosive to Metals H302:Acute toxicity,oral H314:Skin corrosion/irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Phenyl chloroformate
Phenyl chloroformate manufacturer
JSK Chemicals
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Phenyl Chloroformate 98%View Details
Phenyl Chloroformate 98%View Details
1885-14-9 -
 Phenyl Chloroformate CAS 1885-14-9View Details
Phenyl Chloroformate CAS 1885-14-9View Details
1885-14-9 -
 Phenyl Chloroformate CASView Details
Phenyl Chloroformate CASView Details -
 PHENYL CHLOROFORMATE For Synthesis CAS 1885-14-9View Details
PHENYL CHLOROFORMATE For Synthesis CAS 1885-14-9View Details
1885-14-9 -
 Phenyl chloroformate CAS 1885-14-9View Details
Phenyl chloroformate CAS 1885-14-9View Details
1885-14-9 -
 Phenyl chloroformate CAS 1885-14-9View Details
Phenyl chloroformate CAS 1885-14-9View Details
1885-14-9 -
 Phenyl chloroformate CAS 1885-14-9View Details
Phenyl chloroformate CAS 1885-14-9View Details
1885-14-9 -
 Liquid Phenyl ChloroformateView Details
Liquid Phenyl ChloroformateView Details
1885-14-9
