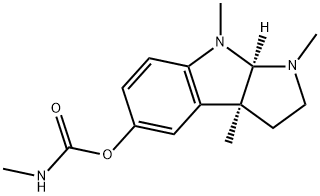
PHENSERINE
Synonym(s):(−)-N-Phenylcarbamoyleseroline;1,2,3,3a,8,8a-Hexahydro-1,3a,8-trimethyl-pyrrolo[2,3-b]indol-5-ol phenylcarbamate (ester)
- CAS NO.:101246-66-6
- Empirical Formula: C20H23N3O2
- Molecular Weight: 337.42
- MDL number: MFCD00672748
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:02:49
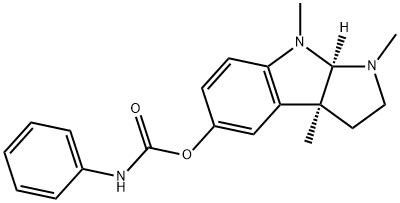
What is PHENSERINE?
The Uses of PHENSERINE
Phenserine is inhibitor of amyloid precursor protein (APP). Used in Alzheimer’s treatments.
What are the applications of Application
Phenserine is inhibitor of amyloid precursor protein (APP)
Biological Activity
Physostigmine analog that inhibits acetylcholinesterase. Inhibits production of amyloid precursor protein (APP) and A β . Improves morris water maze performance of scopolamine-treated rats.
Enzyme inhibitor
This long-acting AChE inhibitor and A?42-lowering agent (FW = 337,42 g/mol; CAS 101246-66-6), also known as (–) -N-phenylcarbamoyl eseroline, is a carbamate analogue of eserine (or physostigmine) that inhibits acetylcholinesterase and is under active investigation for its potential in cholinomimetic therapy to reduce cognitive impairments associated with aging and Alzheimer's Disease. Phenserine is a potent and highly selective AChE inhibitor (IC50 = 22 nM), displaying 70x greater inhibitory action than observed with butyrylcholinesterase, or BChE (IC50 = 1560 nM), both in vitro and in clinical trials for treatment of Alzheimer's disease (1-5). Compared to physostigmine and tacrine, phenserine appears to be less toxic and robustly enhances cognition in animal models. In rats, phenserine achieves maximum acetylcholinesterase inhibition of 73.5% at 5 min, maintaining high and relatively constant inhibition for >8 hours. Phenserine decreased the levels of secreted b-amyloid precursor protein (b- APP) in the cerebrospinal fluid (CSF) of forebrain cholinergic system- lesioned rats, whereas DFP, a relatively non-specific cholinesterase inhibitor, failed to affect CSF levels of secreted b-APP. Such findings suggest that phenserine alters the induction of cortical b-APP mRNAs and increased levels of secreted b-APP in the CSF. Phenserine reduces Ab levels by regulating b-APP translation via a recently described iron regulatory element in the 5'-untranslated region of b-APP mRNA that was previously shown to be up-regulated in the presence of interleukin-1. Other dual AChE/A?42 inhibitors include: rivastigmine, ladostigil, asenapine, phenserine, amitriptyline, clomipramine, doxepin and desipramine. Posiphen, the better-tolerated (+) -enantiomer of phenserine, is devoid of anticholinesterase action, but represses translation of neural α- synuclein, a druggable target in the treatment of Parkinson Disease.
Properties of PHENSERINE
| Melting point: | 151-152 °C |
| Boiling point: | 468.7±45.0 °C(Predicted) |
| Density | 1.228±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | H2O: <2mg/mL |
| form | solid |
| pka | 13.05±0.70(Predicted) |
| color | off-white |
Safety information for PHENSERINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for PHENSERINE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Phenserine CAS 101246-66-6View Details
Phenserine CAS 101246-66-6View Details
101246-66-6 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1