101246-66-6
Product Name:
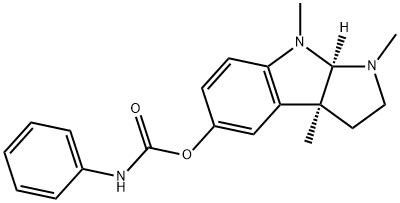
PHENSERINE
Formula:
C20H23N3O2
Synonyms:
(−)-N-Phenylcarbamoyleseroline;1,2,3,3a,8,8a-Hexahydro-1,3a,8-trimethyl-pyrrolo[2,3-b]indol-5-ol phenylcarbamate (ester)
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Melting Point | 150 °C |
|---|
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 337.4 g/mol |
|---|---|
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 337.17902698 g/mol |
| Monoisotopic Mass | 337.17902698 g/mol |
| Topological Polar Surface Area | 44.8 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 507 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Phenserine is under development by Axonyx, a US biopharmaceutical company that focuses on treatments for dementia. Phenserine is a next generation acetylcholinesterase (AChE) inhibitor indicated for the treatment of AD. Unlike currently marketed AChE inhibitors, it has a dual mechanism of action that also includes anti-amyloid activity, which may confer disease-modifying effects in patients with AD. If this is substantiated in an ongoing clinical trial then phenserine may open the door to an entirely new type of treatment for AD. Axonyx announced on 20 September 2005 that phenserine was ineffective in two curtailed phase 3 trials.