Perylene
Synonym(s):Dibenz[de,kl]anthracene;Perylene
- CAS NO.:198-55-0
- Empirical Formula: C20H12
- Molecular Weight: 252.31
- MDL number: MFCD00004142
- EINECS: 205-900-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
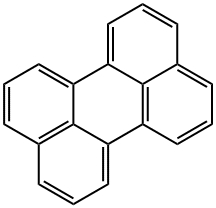
What is Perylene?
Chemical properties
brown solid
The Uses of Perylene
Perylene has been used as an acceptor for pristine C70 to be used as a sensitizer for efficient triplet-triplet annihilation upconversion; which has shown improved stability under continuous laser irradiation compared to the benchmark Pt(II)-ocetaethylprphyrin. Perylene has also been used as a dopant representing blue fluorescent dye on a white-emitting polymer film. Dyes and metabolites.
What are the applications of Application
Perylene is a hydrophobic fluorescent probe
Definition
ChEBI: An ortho- and peri-fused polycyclic arene comprising of five benzene rings that is anthracene in which the d,e and k,l sides are fused to benzene rings.
General Description
Perylene molecules are fluorescent in nature. Ultraviolet photoemission spectroscopy and low energy electron diffraction of the structure of 4? thick perylene dye layers adsorbed on the surface of Ru (0001) has been investigated.
Safety Profile
Questionable carcinogen. Mutation data reported. Whenheated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
Purify perylene by silica-gel chromatography of its recrystallised picrate. [Ware J Am Chem Soc 83 4374 1961.] Crystallise it from *benzene, toluene or EtOH and sublime it at 142o in a flow of oxygen-free nitrogen. It forms a 1:1 *benzene-complex (m 223-224.5o needles from *C6H6), and a 1:2 *benzene-complex (m 154-155o from *C6H6 or H2O). The 2,4,7-trinitrofluoren-9-one has m 270-271o (from EtOH/*C6H6). [Gorman et al. J Am Chem Soc 107 4404 1985, Johansson et al. J Am Chem Soc 109 7374 1987, Beilstein 5 III 2521, 5 IV 2689.]
Properties of Perylene
| Melting point: | 276-279 °C (lit.) |
| Boiling point: | 400 °C |
| Density | 1.35 |
| vapor pressure | <0.001 hPa (20 °C) |
| refractive index | 1.6292 (estimate) |
| Flash point: | >200 °C |
| storage temp. | Store below +30°C. |
| solubility | <0.0001g/l |
| form | Powder |
| color | Ochre to brown |
| Water Solubility | INSOLUBLE |
| Merck | 14,7184 |
| BRN | 1911335 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Combustible. |
| CAS DataBase Reference | 198-55-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Perylene(198-55-0) |
| IARC | 3 (Vol. Sup 7, 92) 2010 |
| EPA Substance Registry System | Perylene (198-55-0) |
Safety information for Perylene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P281:Use personal protective equipment as required. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Perylene
| InChIKey | CSHWQDPOILHKBI-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 198-55-0 Perylene 98%View Details
198-55-0 Perylene 98%View Details
198-55-0 -
 Perylene CAS 198-55-0View Details
Perylene CAS 198-55-0View Details
198-55-0 -
 Perylene 99% (GC CAS 198-55-0View Details
Perylene 99% (GC CAS 198-55-0View Details
198-55-0 -
 Perylene 95% CAS 198-55-0View Details
Perylene 95% CAS 198-55-0View Details
198-55-0 -
 Perylene (purified by sublimation) CAS 198-55-0View Details
Perylene (purified by sublimation) CAS 198-55-0View Details
198-55-0 -
 Perylene CAS 198-55-0View Details
Perylene CAS 198-55-0View Details
198-55-0 -
 Perylene CAS 198-55-0View Details
Perylene CAS 198-55-0View Details
198-55-0 -
 Perylene CAS 198-55-0View Details
Perylene CAS 198-55-0View Details
198-55-0
