Anthracene
Synonym(s):Anthracene;Anthraxcene;Paranaphthalene
- CAS NO.:120-12-7
- Empirical Formula: C14H10
- Molecular Weight: 178.23
- MDL number: MFCD00001240
- EINECS: 204-371-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
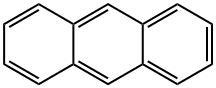
What is Anthracene?
Description
Anthracene is one of a group of chemicals called polycyclic aromatic hydrocarbons (PAHs). PAHs are often found together in groups of two ormore. They can exist inmore than 100 different combinations, but the most common are treated as a group of 15. PAHs are found naturally in the environment but they can also be made synthetically. Anthracene can vary in appearance from a colorless to pale yellow crystal-like solid. PAHs are created when products like coal, oil, gas, and garbage are burned but the burning process is not complete. Very little information is available on the individual chemicals within the PAH group; the majority of the information is for the entire PAH group. Anthracene is a solid white to yellow crystal, has a weak aromatic odor, and sinks in water. Its characteristics are boiling point, 3421°C; melting point, 2181°C; molecular weight, 178.22; density/specific gravity, 1.25 at 27 and 41°C; octanol–water coefficient, 4.45. It is soluble in absolute alcohol and organic solvents. Maximum absorption occurs at 218 nm.
Description
Anthracene is one of the smaller polynuclear aromatic hydrocarbons. (The only smaller one is naphthalene.) It was isolated from coal tar in 1832 by pioneering French chemists Jean-Baptiste Dumas and Auguste Laurent.
Anthracene can be synthesized by the Elbs reaction, in which o-tolyl phenyl ketone is dehydrated at 400–450 oC. But most commercial anthracene is still recovered from coal tar.
In commerce, anthracene is mainly used as a starting material for the manufacture of 9,10-anthraquinone, which in turn is used to make colorants such as the red dye alizarin. More recently, crystalline anthracene was found to be a useful wide band-gap semiconductor in devices such as organic field-effect transistors and scintillators for detecting high-energy subatomic particles.
Chemical properties
Anthracene is colorless, to pale yellow crystalline solid with a bluish fluorescence. PAHs are compounds containing multiple benzene rings and are also called polynuclear aromatic hydrocarbons.
Chemical properties
ANTHRACENE is a colorless solid; melting point 218 °C, blue fluorescence when pure; insoluble in water, slightly soluble in alcohol or ether, soluble in hot benzene, slightly soluble in cold benzene; transformed by sunlight into para -anthracene (C14H10)2.
Physical properties
White to yellow crystalline flakes or crystals with a bluish or violet fluorescence and a weak aromatic odor. Impurities (naphthacene, tetracene) impart a yellowish color with green fluorescence.
The Uses of Anthracene
Anthracene is an aromatic hydrocarbonwith three fused rings, and is obtained by the distillationof crude oils. The main useis in the manufacture of dyes.It is an important source of dyestuffs.
The Uses of Anthracene
Anthracene has been shown to be soluble in a variety of binary and ternary mixtures of cyclohexanone, ethyl acetate, and methanol 1,2.
The Uses of Anthracene
Most of the PAHs are used to conduct research. Like most PAHs, anthracene is used to make dyes, plastics, and pesticides. It has been used to make smoke screens and scintillation counter crystals. (A scintillation counter is used to detect or count the number of sparks or flashes that occur over a period of time.)
What are the applications of Application
Anthracene is a polycyclic aromatic hydrocarbon that fluoresces blue under UV light
Definition
(C14H10) A white crystalline solid used extensively in the manufacture of dyes. Anthracene is found in the heavy- and green-oil fractions of crude oil and is obtained by fractional crystallization. Its structure is benzene-like, having three six-membered rings fused toanion gether. The reactions are characteristic of AROMATIC COMPOUNDS.
Production Methods
Anthracene is obtained from coal tar in the fraction distilling between 300° and 400 °C. This fraction contains 5–10% anthracene, from which, by fractional crystallization followed by crystallization from solvents, such as oleic acid, and washing with such solvents as pyridine, relatively pure anthracene is obtained. It may be detected by the formation of a blue-violet coloration on fusion with mellitic acid. Anthracene derivatives, especially anthraquinone, are important in dye chemistry.
Reactions
Anthracene reacts: (1) With oxidizing agents, e.g., sodium dichromate plus sulfuric acid, to form anthraquinone, C6H4(CO)2C6H. (2) With chlorine in water or in dilute acetic acid below 250 °C to form anthraquinol and anthraquinone, at higher temperatures 9,10-dichloroanthracene. The reaction varies with the temperature and with the solvent used. The reaction has been studied using, as solvent, benzene, chloroform, alcohol, carbon disulfide, ether, glacial acetic acid, and also without solvent by heating. Bromine reacts similarly to chlorine. (3) With concentrated sulfuric acid to form various anthracene sulfonic acids. (4) With nitric acid, to form nitroanthracenes and anthraquinone. (5) With picric acid (1)HO·C6H2(NO2)3(2,4,6) to form red crystalline anthracene picrate, melting point 138 °C.
Synthesis Reference(s)
Journal of the American Chemical Society, 82, p. 3653, 1960 DOI: 10.1021/ja01499a046
Synthetic Communications, 7, p. 161, 1977 DOI: 10.1080/00397917708050729
Tetrahedron Letters, 35, p. 1131, 1994 DOI: 10.1016/0040-4039(94)88004-2
General Description
White to yellow solid with a weak aromatic odor. Sinks in water.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
Anthracene will spontaneously burst into flame on contact with chromic acid, and other strong oxidants.
Hazard
A questionable carcinogen.
Health Hazard
Carcinogenicity of anthracene is not known.Its toxicity is very low. An intraperitonealLD50 in mice is recorded at 430 mg/kg(NIOSH 1986).
Health Hazard
Inhalation of dust irritates nose and throat. Contact with eyes causes irritation.
Fire Hazard
Anthracene is combustible.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by intraperitoneal route. A skin irritant and allergen. Questionable carcinogen with experimental neoplas tigenic and tumorigenic data. Mutation data reported. Combustible when exposed to heat, flame, or oxidizing materials. Moderately explosive when exposed to flame, Ca(OCl)z, chromic acid. To fight fire, use water, foam, CO2, water spray or mist, dry chemical. Explodes on contact with fluorine.
Toxicology
As a polycyclic aromatic hydrocarbon,
anthracene is suspected to be carcinogenic
. This earlier
experience involving workers is based on crude
anthracene that was contaminated with various
other polycyclic aromatic hydrocarbons. Pure
anthracene, however, has no appreciable carcinogenic
effect under experimental conditions
. This is underlined by consistently
negative findings in numerous in vitro and in
vivo genotoxicity tests . Only subcutaneous
injections of an oily solution containing
20 mg of anthracene, given 33 times at the rate
of one per week, resulted in local development
of fibroma, to some extent with sarcoma-like excrescences
. An epicutaneous tumor initiation
test conducted over 35weeks on mice with pure anthracene and phorbol ester as promoter
resulted in papilloma in a few cases (4
out of 28 animals) . Anthracene is classified
as “not classifiable as to its carcinogenicity
to humans” by IARC (Category 3) and by EPA
(Group D) .
Peroral application of 1.7 g/kg of pure anthracene
has no lethal effect on mice . Anthracene
is absorbed percutaneously: after topical
application of a 14C-labeled solution in hexane
or acetone (ca. 9 μg/cm3) to rat skin, some
50% was absorbed in 6 d (ca. 29% was recovered
from the urine, ca. 22% from the feces,
and ca. 1% from tissue, mainly the liver and
kidneys); after 1 d 20% of the dose was already
present in the urine (ca. 17 %) and feces (ca.
3%) . Anthracene can sensitize the skin locally
to light . Gerarde proposes a TLV of
0.1 mg/m3 .
Synthesis
Anthracene can be synthesized by hydrogenation of phenanthrene, isomerization of the resulting sym-octahydrophenanthrene to symoctahydroanthracene, and subsequent dehydrogenation .Amixture of sym-octahydroanthracene and sym-octahydrophenanthrene is obtained by catalytic disproportionation of tetralin . In addition, anthracene is formed from diphenylmethane in the presence of HF/BF3 at 80 ?C and by thermal reaction of o-methyldiphenylmethane at ca. 600 ?C .
Potential Exposure
It is used as an intermediate in dye stuffs (alizarin), insecticides, and wood preservatives; making synthetic fibers, anthraquinone, and other chemicals. May be present in coke oven emissions, diesel fuel, and coal tar pitch volitiles.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
Anthracene was negative in mouse-skin-painting studies, and it is classified as a noncarcinogen by the IARC based on inadequate evidence. The methyl, anthryl, dimethyl, diprophyl, dinaphthyl, and tetramethyl derivatives of anthracene were noncarcinogenic except for 9,10-dimethyl anthracene, which may have contained impurities when tested.
Source
Concentrations in 8 diesel fuels ranged from 0.026 to 40 mg/L with a mean value of
6.275 mg/L (Westerholm and Li, 1994). Lee et al. (1992) reported concentration ranges of 100–
300 mg/L and 0.04–2 μg/L in diesel fuel and corresponding aqueous phase (distilled water),
respectively. Schauer et al. (1999) reported anthracene in diesel fuel at a concentration of 5 μg/g
and in a diesel-powered medium-duty truck exhaust at an emission rate of 12.5 μg/km. Anthracene
was detected in a distilled water-soluble fraction of used motor oil at concentrations ranging from
1.1 to 1.3 μg/L (Chen et al., 1994).
California Phase II reformulated gasoline contained anthracene at a concentration of 4.35 μg/kg.
Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without catalytic
converters were 3.69 and 148 μg/km, respectively (Schauer et al., 2002).
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
625. Average anthracene concentrations reported in water-soluble fractions of kerosene and diesel
fuel were 12 and 25 μg/L, respectively. Anthracene was ND in the water-soluble fraction of
unleaded gasoline.
The concentration of anthracene in coal tar and the maximum concentration reported in
groundwater at a mid-Atlantic coal tar site were 5,000 and 0.02 mg/L, respectively (Mackay and
Gschwend, 2001). Based on laboratory analysis of 7 coal tar samples, anthracene concentrations
ranged from 400 to 8,600 ppm (EPRI, 1990). A high-temperature coal tar contained anthracene at
an average concentration of 0.75 wt % (McNeil, 1983). Lehmann et al. (1984) reported an
anthracene concentration of 34.8 mg/g in a commercial anthracene oil.
Nine commercially available creosote samples contained anthracene at concentrations ranging
from 5,500 to 14,000 mg/kg (Kohler et al., 2000).
Anthracene was detected in asphalt fumes at an average concentration of 45.89 ng/m3 (Wang et
al., 2001).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The respective gas-phase
and particle-phase emission rates of anthracene were 3.44 and 0.228 mg/kg of pine burned, 2.13
and 0.0230 mg/kg of oak burned, and 1.76 and 0.0061 mg/kg of eucalyptus burned.
Under atmospheric conditions, a low rank coal (0.5–1 mm particle size) from Spain was burned
in a fluidized bed reactor at seven different temperatures (50 °C increments) beginning at 650 °C.
The combustion experiment was also conducted at different amounts of excess oxygen (5 to 40%)
and different flow rates (700 to 1,100 L/h). At 20% excess oxygen and a flow rate of 860 L/h, the
amount of anthracene emitted ranged from 558.7 ng/kg at 900 °C to 2,449.7 ng/kg at 800 °C. The
greatest amount of PAHs emitted were observed at 750 °C (Mastral et al., 1999).
Environmental Fate
Biological. Catechol is the central metabolite in the bacterial degradation of anthracene.
Intermediate by-products included 3-hydroxy-2-naphthoic acid and salicylic acid (Chapman,
1972). Anthracene was statically incubated in the dark at 25 °C with yeast extract and settled
domestic wastewater inoculum. Significant biodegradation with gradual adaptation was observed.
At concentrations of 5 and 10 mg/L, biodegradation yields at the end of 4 wk of incubation were
92 and 51%, respectively (Tabak et al., 1981). A mixed bacterial community isolated from
seawater foam degraded anthraquinone, a photodegradation product of anthracene, to traces of
benzoic and phthalic acids (Rontani et al., 1975). In activated sludge, only 0.3% mineralized to
carbon dioxide after 5 d (Freitag et al., 1985).
Soil. In a 14-d experiment, [14C]anthracene applied to soil-water suspensions under aerobic and
anaerobic conditions gave 14CO2 yields of 1.3 and 1.8%, respectively (Scheunert et al., 1987). The
reported half-lives for anthracene in a Kidman sandy loam and McLaurin sandy loam are 134 and
50 d, respectively (Park et al., 1990).
Surface Water. The removal half-lives for anthracene in a water column at 25 °C in midsummer
sunlight were 10.5 h for deep, slow, slightly turbid water; 21.6 h for deep, slow, muddy water; 8.5
h deep, slow, clear water; 3.5 h for shallow, fast, clear water, and 1.4 h for very shallow, fast, clear
water (Southworth, 1977).
Photolytic. Oxidation of anthracene adsorbed on silica gel or alumina by oxygen in the presence
of UV-light yielded anthraquinone. This compound additionally oxidized to 1,4-dihydroxy-
9,10-anthraquinone. Anthraquinone also formed by the oxidation of anthracene in diluted nitric
acid or nitrogen oxides (quoted, Nikolaou et al., 1984) and in the dark when adsorbed on fly ash
(Korfmacher et al., 1980). Irradiation of anthracene (2.6 mM) in cyclohexanone solutions gave
9,10-anthraquinone as the principal product (Korfmacher et al., 1980). Photocatalysis of
anthracene and sulfur dioxide at -25 °C in various solvents yielded anthracene-9-sulfonic acid
(Nielsen et al., 1983). Schwarz and Wasik (1976) reported a fluorescence quantum yield of 0.25
for anthracene in water.
Chemical/Physical. In urban air from St. Louis, MO, anthracene reacted with NOx forming 9-
nitroanthracene (Ramdahl et al., 1982).
Storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Before entering confined space where this chemical may bepresent, check to make sure that an explosive concentrationdoes not exist. Anthracene must be stored to avoid contactwith strong oxidizers (such as chlorine, bromine, and fluorine), chromic acid, and calcium hypochlorite, since violentreactions occur. Store in tightly closed containers in a cool,well-ventilated area. Sources of ignition, such as smokingand open flames, are prohibited where Anthracene is used,handled, or stored in a manner that could create a potentialfire or explosion hazard.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Likely impurities are anthraquinone, anthrone, carbazole, fluorene, 9,10-dihydroanthracene, tetracene and bianthryl. Carbazole is removed by continuous-adsorption chromatography [see Sangster & Irvine J Phys Chem 24 670 1956] using a neutral alumina column and eluting with n-hexane. [Sherwood in Purification of Inorganic and Organic Materials, Zief (ed), Marcel Dekker, New York, 1969.] The solvent is evaporated, and anthracene is sublimed under vacuum, then purified by zone refining, under N2 in darkness or non-actinic light. It has also been purified by co-distillation with ethylene glycol (boils at 197.5o), from which it can be recovered by addition of water, followed by crystallisation from 95% EtOH, *benzene, toluene, a mixture of *benzene/xylene (4:1), or Et2O. It has also been chromatographed on alumina with pet ether in a dark room (to avoid photo-oxidation of adsorbed anthracene to anthraquinone). Other purification methods include sublimation in a N2 atmosphere (in some cases after refluxing with sodium), and recrystallisation from toluene [Gorman et al. J Am Chem Soc 107 4404 1985]. Anthracene has been crystallised from EtOH, chromatographed through alumina in hot *benzene (fume hood) and then sublimed in a vacuum in a pyrex tube that has been cleaned and baked at 100o. (For further details see Craig & Rajikan J Chem Soc, Faraday Trans 1 74 292 1978, and Williams & Zboinski J Chem Soc, Faraday Trans 1 74 611 1978.) It has been chromatographed on alumina, recrystallised from n-hexane and sublimed under reduced pressure. [Saltiel J Am Chem Soc 108 2674 1986, Masnori et al. J Am Chem Soc 108 1126 1986.] Alternatively, recrystallise it from cyclohexane, chromatograph it on alumina with n-hexane as eluent, and recrystallise two more times [Saltiel et al. J Am Chem Soc 109 1209 1987]. Anthracene is fluorescent and forms a picrate complex, m 139o, on mixing the components in CHCl3 or *C6H6, but decomposes on attempted crystallization. [Beilstein 5 IV 228.]
Incompatibilities
Finely dispersed powder may form explosive mixture in air. Contact with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, chromic acid/or calcium hypochlorite.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration.
Properties of Anthracene
| Melting point: | 210-215 °C (lit.) |
| Boiling point: | 340 °C (lit.) |
| Density | 1.28 |
| vapor density | 6.15 (vs air) |
| vapor pressure | 1 mm Hg ( 145 °C) |
| refractive index | 1.5948 |
| Flash point: | 121 °C |
| storage temp. | 2-8°C |
| solubility | toluene: soluble20mg/mL, clear, colorless to faintly yellow |
| appearance | white crystals or powder |
| form | powder |
| Colour Index | 10790 |
| pka | >15 (Christensen et al., 1975) |
| color | off-white to yellow |
| explosive limit | 0.6%(V) |
| Water Solubility | 0.045 mg/L (25 ºC) |
| Merck | 14,682 |
| BRN | 1905429 |
| Henry's Law Constant | 1.22 at 4 °C, 6.42 at 25 °C (dynamic equilibrium method, Bamford et al., 1999) |
| Exposure limits | OSHA: TWA 0.2 mg/m3 |
| Dielectric constant | 2.3500000000000001 |
| CAS DataBase Reference | 120-12-7(CAS DataBase Reference) |
| IARC | 3 (Vol. 92, Sup 7) 2010 |
| NIST Chemistry Reference | Anthracene(120-12-7) |
| EPA Substance Registry System | Anthracene (120-12-7) |
Safety information for Anthracene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Anthracene
Anthracene manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Anthracene, for scintillation CAS 120-12-7View Details
Anthracene, for scintillation CAS 120-12-7View Details
120-12-7 -
 Anthracene, 99% CAS 120-12-7View Details
Anthracene, 99% CAS 120-12-7View Details
120-12-7 -
 Anthracene extrapure CAS 120-12-7View Details
Anthracene extrapure CAS 120-12-7View Details
120-12-7 -
 Anthracene, 96% CAS 120-12-7View Details
Anthracene, 96% CAS 120-12-7View Details
120-12-7 -
 Anthracene (High Purity) scintillation grade CAS 120-12-7View Details
Anthracene (High Purity) scintillation grade CAS 120-12-7View Details
120-12-7 -
 Anthracene Cas No:120-12-7View Details
Anthracene Cas No:120-12-7View Details
120-12-7 -
 ANTHRACENE 96 %View Details
ANTHRACENE 96 %View Details
120-12-7 -
 Off White to Light Green Anthracene (CAS Number: 120-12-7)View Details
Off White to Light Green Anthracene (CAS Number: 120-12-7)View Details
120-12-7
