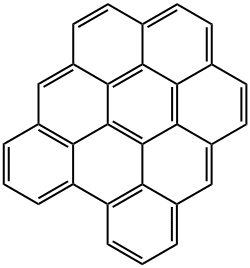
1.12,2.3,4.5,6.7,8.9,10.11-HEXABENZOCORONENE
- CAS NO.:190-24-9
- Empirical Formula: C42H18
- Molecular Weight: 522.59
- MDL number: MFCD00049075
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-25 20:04:51
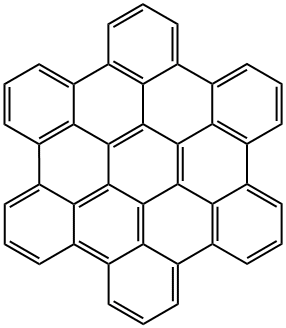
What is 1.12,2.3,4.5,6.7,8.9,10.11-HEXABENZOCORONENE?
Description
Hexa-peri-benzocoronene (HBC), or, more commonly, hexabenzocoronene, is a planar polycyclic aromatic hydrocarbon (PAH). Structurally, it consists of a central benzene ring surrounded first by six more benzene rings to form?coronene1, which in turn is surrounded by six additional benzenes for a total of 13 rings. HBC is in the?D6h?symmetry group.
Two HBC syntheses appeared in 1958. In one, A. Halleux, R. H. Martin, and G. S. D. King at European Research Associates (Brussels)?used two reactions?to make the molecule: the cyclodehydrogenation of hexaphenylbenzene2?and the reaction of dibenz-1,9;2,3-anthrone3?with Zn/ZnCl2. They also isolated two new hydrocarbons from the Zn/ZnCl2?reaction.
In the other synthesis, E. Clar and C. T. Ironside at the University of Glasgow first treated 2,3,7,8-dibenzoperinaphthene with bromine, then heated the brominated product to form tetrabenzoperopyrene4?(TBP) Additional heating?dehydrogenated TBP to produce HBC. The authors also reported that HBC emits orange-yellow phosphorescence in solid solution at low temperatures.
In 2014, Andreas Hirsch and collaborators at the University of Erlangen–Nuremberg (Germany) synthesized a compound in which?HBC is covalently linked to buckminsterfullerene5. The authors stated that this “dyad” could serve as a versatile template for optoelectronic applications. X-ray crystallography showed that the bond distance between the two aromatic components is only 3.17 ?.
In another example of HBC and its derivatives combined with other chemical structures, Aleksey E. Kuznetsov at Federico Santa María Technical University (Santiago, Chile)?paired HBCs with antiaromatic sulfur- and selenium-core-modified porphyrins?(isophlorins). In this theoretical study, the author found that isophlorins had profound effects on the structural and electronic properties of the dyads and therefore likely their reactivities.
Although PAHs similar to HBC are known carcinogens, there are no reports in the literature of its carcinogenicity.
1. CAS Reg. No. 191-07-1.
2. CAS Reg. No. 992-04-1.
3. CAS Reg. No. 86854-05-9.
4. CAS Reg. No. 190-22-7.
5. CAS Reg. No. 99685-96-8; MOTW for?June 11, 2018.
Properties of 1.12,2.3,4.5,6.7,8.9,10.11-HEXABENZOCORONENE
| Melting point: | >600 °C |
| Density | 1.633±0.06 g/cm3(Predicted) |
| solubility | insoluble |
| appearance | yellow to orange-yellow crystals or powder |
Safety information for 1.12,2.3,4.5,6.7,8.9,10.11-HEXABENZOCORONENE
Computed Descriptors for 1.12,2.3,4.5,6.7,8.9,10.11-HEXABENZOCORONENE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![NAPHTHO[1,2,3,4-GHI]PERYLENE](https://img.chemicalbook.in/CAS/GIF/190-84-1.gif)
![Dibenz[a,h]anthracene](https://img.chemicalbook.in/CAS/GIF/53-70-3.gif)
![BENZO[G]CHRYSENE](https://img.chemicalbook.in/CAS/GIF/196-78-1.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1