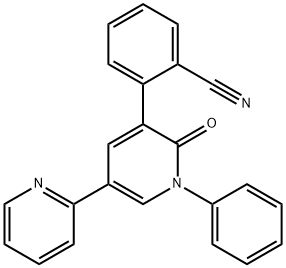
PeraMpanel
- CAS NO.:380917-97-5
- Empirical Formula: C23H15N3O
- Molecular Weight: 349.38
- MDL number: MFCD19443693
- EINECS: 691-969-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-04 09:00:37
What is PeraMpanel?
Absorption
After oral adminitration, perampanel is absorbed rapidly and completely.
Toxicity
The FDA label includes an important warning of serious or life-threatening behavioral and psychiatric adverse reactions including aggression, hostility, irritability, anger, and homicidal thoughts in patients taking perampanel.
Description
In October 2012, the US FDA approved perampanel for the treatment of partial onset seizures in epileptic patients who are at least 12 years old. Perampanel is the first AMPA receptor antagonist to receive FDA approval as an AED. AMPA glutamate receptors are found primarily on postsynaptic neurons in the brain. As a selective, noncompetitive antagonist of AMPA, parampanel prevents ion channel opening and reduces propagation of action potential. Parampanel was discovered through lead optimization of a commercially available compound, 2,4-diphenyl-4H-[1,3,4]oxadiazin-5-one, which was identified by high-throughput screening of a compound collection employing a rat cortical neuron AMPA-induced cell-death assay. Modifications of aromatic rings at positions 1, 3, and 5 while changing the core to pyridone led to parampanel which inhibited AMPA-induced calcium influx (IC50=60 nM). Parampanel had a minimum effective oral dose of 2 mg/kg in an AMPA-induced mouse seizure model. The synthesis of parampanel was accomplished via a 6-step route utilizing Suzuki–Miyaura couplings and modified Ullmann reactions for incorporation of aryl groups.
Originator
Eisai (Japan)
The Uses of PeraMpanel
Isotope labelled Perampanel (P285520), is an antiepileptic drug. It inhibits α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA)-induced increases in intracellular Ca2+ and selectively blocks AMPA receptor-mediated synaptic transmission, thus reducing neuronal excitation.
Background
Perampanel is a noncompetitive AMPA glutamate receptor antagonist. It is marketed under the name Fycompa? and is indicated as an adjunct in patients over 12 years old for the treatment of partial-onset seizures that may or may not occur with generalized seizures. The FDA label includes an important black-boxed warning of serious or life-threatening behavioral and psychiatric reactions in patients taking Fycompa?.
Indications
Perampanel is indicated for the treatment of partial-onset seizures with or without secondarily generalized seizures in epileptic patients four years of age and older. It is also indicated as an adjunct in the treatment of primary generalized tonic-clonic seizures in epileptic patients aged 12 years and older.
Definition
ChEBI: A member of the class of bipyridines that is 2,3'-bipyridin-6'-one substituted at positions 1' and 5' by phenyl and 2-cyanophenyl groups respectively. Used as an adjunctive therapy for the treatment of partial-onset seizures in patients with epilepsy.
brand name
Fycompa
Pharmacokinetics
Perampanel is involved in inhibiting neuronal excitation in the central nervous system leading to such effects as decreased pyschomotor performance.
Clinical Use
Selective AMPA-type glutamate receptor antagonist:
Antiepileptic
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: anticonvulsant effect antagonised;
avoid with St John’s wort.
Antiepileptics: concentration reduced by
carbamazepine, fosphenytoin, oxcarbazepine and
phenytoin.
Antimalarials: anticonvulsant effect antagonised by
mefloquine.
Antipsychotics: anticonvulsant effect antagonised.
Orlistat: possibly increased risk of convulsions.
Progestogens: high-dose perampanel reduces plasma
concentration of progestogens (possibly reduced
contraceptive effect).
Metabolism
Perampanel is highly metabolized by CYP3A4 and/or CYP3A5 primary oxidation and by sequential glucuronidation.
Metabolism
Extensively metabolised via primary oxidation via the
cytochrome P450 isoenzyme CYP3A sub family and
sequential glucuronidation.
Perampanel is excreted in the urine and faeces mainly as
oxidative and conjugated metabolites.
Properties of PeraMpanel
| Boiling point: | 619.1±55.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | 4.73±0.19(Predicted) |
Safety information for PeraMpanel
Computed Descriptors for PeraMpanel
PeraMpanel manufacturer
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran

You may like
-
 Perampanel 98%View Details
Perampanel 98%View Details -
 380917-97-5 99%View Details
380917-97-5 99%View Details
380917-97-5 -
 Perampanel 98%View Details
Perampanel 98%View Details
380917-97-5 -
 Perampanel 380917-97-5 98%View Details
Perampanel 380917-97-5 98%View Details
380917-97-5 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3 -
 73590-58-6 Omeprazole 98%View Details
73590-58-6 Omeprazole 98%View Details
73590-58-6 -
 Sertraline HCl 98%View Details
Sertraline HCl 98%View Details
79559-97-0
