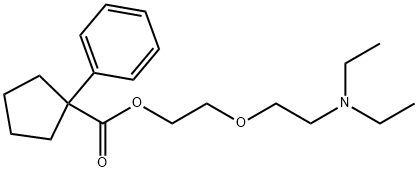
Pentoxyverine
- CAS NO.:77-23-6
- Empirical Formula: C20H31NO3
- Molecular Weight: 333.46
- MDL number: MFCD00209899
- EINECS: 201-014-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
What is Pentoxyverine?
Absorption
In humans, maximum plasma concentrations are achieved 1.2 hours after oral dosing .
Toxicity
Acute oral LD50 is 810 mg/kg in rat and 230 mg/kg in mouse .
Originator
Asthma ,Nichiiko
The Uses of Pentoxyverine
Carbetapentane is an antitussive anticonvulsant sigma site agonist.
Indications
Indicated as a cough suppressant to relieve cough caused by the common cold, flu, bronchitis, and sinusitis .
Background
Pentoxyverine, also referred to as carbetapentane, is a non-opioid central acting antitussive with antimuscarinic, anticonvulsant , and local anesthetic properties. It is an active ingredient in over-the-counter cough suppressants in combination with guaifenesin and H1-receptor antagonists . Pentoxyverine acts on sigma-1 receptors, as well as kappa and mu-opioid receptors.
The FDA withdrew the use of all oral gel drug products containing pentoxyverine citrate. Other forms of pentoxyverine citrate continue to be marketed.
What are the applications of Application
Carbetapentane is an antitussive anticonvulsant sigma site agonist
Definition
ChEBI: 1-phenyl-1-cyclopentanecarboxylic acid 2-[2-(diethylamino)ethoxy]ethyl ester is a member of benzenes.
Manufacturing Process
1-Phenylcyclopentane carbonitrile was obtained by treatment of phenylacetonitrile with sodium amide and 1,4-dibrombutane.
1-Phenyl-1-cyclopentane carboxylic acid was produced in the result of reaction of 1-phenylcyclopentane carbonitrile with sulfuric acid.
1-Phenyl-1-cyclopentanecarbonyl chloride was obtained by treatment of 1phenyl-1-cyclopentane carboxylic acid with thionyl chloride.
A mixture of 0.5 mol of 1-phenyl-1-cyclopentanecarbonyl chloride and of 0.5 mol of 2-(2-diethylaminoethoxy)ethanol (herein-after referred to as the amino alcohol) in 300 ml of toluene is heated under reflux for 20 h. The mixture is thereafter made alkaline by means of an aqueous solution of caustic soda and decanted; the toluenic layer is washed with water and concentrated in vacuo. The residue is distilled under high vacuum. After two fractional distillations, the 2-(2-diethylaminoethoxy)ethyl 1-phenylcyclopentane-carboxylate is obtained, in 85% yield. Boiling point 164°C/0.1 mm. Hg.
brand name
Toclase (Pfizer).
Therapeutic Function
Antitussive
Pharmacokinetics
Pentoxyverine induces an antitussive action. In animal studies, intraperitoneal administration of pentoxyverine inhibited citric-acid-induced cough in guinea-pigs in vivo . Some mice and rat studies suggest that pentoxyverine may also exert anticonvulsant activities without inducing a protective effect from NMDA-induced lethality . Protective effects against maximal electroshock-induced seizures in a dose-related fashion was also observed following either intraperitoneal or oral administration . In hERG-transfected cells, pentoxyverine inhibited the outward current of the hERG ion channel with half-maximal inhibition concentrations (IC50) of 3.0 μM . In rats receiving intrathecal administration, pentoxyverine exhibited dose-dependent spinal blockade with a more sensory-selective action over motor blockade . It induced a spinal blockade with a more sensory/nociceptive-selective action over motor blockade compared to lidocaine .
Metabolism
No pharmacokinetic data available.
Properties of Pentoxyverine
| Boiling point: | 165-170 °C (1.33Pa) |
| Density | 1.048±0.06 g/cm3(Predicted) |
| refractive index | 1.4990-1.5010 |
| storage temp. | Refrigerator |
| solubility | Dichloromethane (Slightly), Methanol (Slightly) |
| form | Oil |
| pka | 9.69±0.25(Predicted) |
| color | Clear Very Dark Red to Brown |
| CAS DataBase Reference | 77-23-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Cyclopentanecarboxylate, 2-(diethylaminoethoxy)ethyl-1-phenyl-(77-23-6) |
Safety information for Pentoxyverine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pentoxyverine
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1