Penciclovir
Synonym(s):2-Amino-1,9-dihydro-9-[4-hydroxy-3-(hydroxymethyl)butyl]-6H-purin-6-one;BRL-39123;Penciclovir - CAS 39809-25-1 - Calbiochem
- CAS NO.:39809-25-1
- Empirical Formula: C10H15N5O3
- Molecular Weight: 253.26
- MDL number: MFCD00866931
- EINECS: 663-371-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
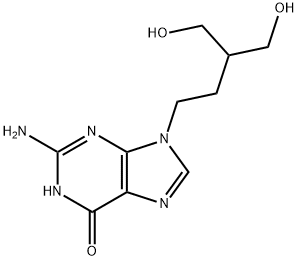
What is Penciclovir?
Absorption
Measurable penciclovir concentrations were not detected in plasma or urine of healthy male volunteers (n= 12) following single or repeat application of the 1% cream at a dose of 180 mg penciclovir daily.
Toxicity
Symptoms of overdose include headache, abdominal pain, increased serum lipase, nausea, dyspepsia, dizziness, and hyperbilirubinemia.
Description
Vectavir was launched in the UK for herpes labialis. Penciclovir is synthetically available by two routes of four steps each from 2- (hydroxymethyl)butane-l,4-diol and is active against HSV-1, HSV-2 VZV but has limited activity against CMV. Vectavir is an acyclic guanosine analog that acts as a competitive inhibitor of DNA polymerase. It is a metabolic product of famcyclovir that is preferentially phosphorylated by viral infected cells (by thymidine kinases) over normal cells. The triphosphate has a low activity against cellular DNA polymerase which is one possible explanation for its low toxicity. While its spectrum of activity is similar to acyclovir, it is longer acting because its triphosphate is 20 times more stable and is not metabolized.
Chemical properties
White Cyrstalline Solid
Originator
SmithKline Beecham (UK)
The Uses of Penciclovir
A deuterated version of Penciclovir, an antiviral
The Uses of Penciclovir
An antiviral.
Background
Penciclovir is a synthetic acyclic guanine derivative with antiviral activity used for the treatment of various herpes simplex virus (HSV) infections. Displaying low toxicity and good selectivity, penciclovir is a nucleoside analogue.
Indications
Used to treat recurrent cold sores on the lips and face from various herpesvirus invections.
What are the applications of Application
Penciclovir is a purine acyclic nucleoside analogue with potent antiviral activity
Definition
ChEBI: A member of the class of 2-aminopurines that is guanine in which the hydrogen at position 9 is substituted by a 4-hydroxy-3-(hydroxymethyl)but-1-yl group. An antiviral drug, it is administered topically for treatment of herpes labialis. A prodrug, famciclo ir, is used for oral administration.
Indications
Penciclovir has activity against HSV-1, HSV-2, VZV, and HBV. After oral administration, famciclovir is converted to penciclovir by first-pass metabolism. Penciclovir has a mechanism of action similar to that of acyclovir. It is first monophosphorylated by viral thymidine kinase; then it is converted to a triphosphate by cellular kinases.
Manufacturing Process
To a suspension of lithium aluminum hydride (2.87 g, 76 mmol) in tetrahydrofuran (125 ml), a solution of triethyl 1,1,2-ethanetricarboxylate (9.2 ml, 9.85 g, 40 mmol) in tetrahydrofuran (25 ml) was added dropwise with stirring over 2 hours. The inorganic salts were filtered off and washed with ethanol (100 ml). The filtrate and washings were combined and the solvent was evaporated under reduced pressure to afford a colourless oil (4.85 g). To a suspension of this oil in acetone (100 ml) 2,2-dimethoxypropane (25 ml) and p-toluenesulphonic acid monohydrate (2.3 g, 12 mmol) were added. The mixture was stirred for 1 hour. The resulting solution was neutralised with Amberlite IR 45 (methanol washed), filtered and the solvent evaporated under reduced pressure. The residue was purified by column chromatography on silica gel, eluting with chloroform-methanol mixtures (40:1 and 25:1) to afford 5-(2-hydroxyethyl)-2,2-dimethyl-1,3-dioxan as a colourless liquid (3.01 g, 47%).
To an ice-cooled solution of 5-(2-hydroxyethyl)-2,2-dimethyl-1,3-dioxan (1.92 g, 12 mmol) and carbon tetrabromide (7.96 g, 24 mmol) in dimethylformamide (100 ml) triphenylphosphine (6.30 g, 24 mmol) was added and the solution was left at 4°C overnight. To this solution methanol (20 ml) was added and the solvent was then evaporated under reduced pressure. The residue was purified by column chromatography on silica gel, eluting with hexane-acetone (12:1) to afford 5-(2-bromoethyl)-2,2-dimethyl1,3-dioxan as a clear colourless liquid (0.89 g, 40%).
To a solution of 5-(2-bromoethyl)-2,2-dimethyl-1,3-dioxan (0.75 g, 3.7 mmol) in dry dimethylformamide (12 ml) 2-amino-6-chloropurine (0.68 g, 4.0 mmol) and then anhydrous potassium carbonate (0.83, 6.0 mmol) were added. The solution was stirred at room temperature for 5 hours and left at 4°C
overnight. The solution was filtered and the solvent removed. The residue was purified by column chromatography on silica gel, eluting with chloroformmethanol mixtures (80:1 and 60:1) to afford 2-amino-6-chloro-9-[2-(2,2dimethyl-1,3-dioxan-5-yl)ethyl]purine as a white crystalline solid (0.74 g, 64%), melting point 125°-126°C.
2-Amino-6-chloro-9-[2-(2,2-dimethyl-1,3-dioxan-5-yl)-ethyl]purine (0.59 g, 1.9 mmol) in hydrochloric acid (1.0 M, 4 ml) was stirred at 60°C for 24 hours. The solution was diluted with water and neutralised with Amberlite IR 45. The mixture was filtered, the resin washed with water and the solvent evaporated under reduced pressure. The residue was recrystallised from water to afford 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (238 mg, 49%), melting point 275°-277°C.
brand name
Denavir (SmithKline Beecham).
Therapeutic Function
Antiviral
Acquired resistance
Penciclovir is inactive against thymidine kinase-deficient strains of HSV.
Pharmaceutical Applications
A synthetic acyclic purine nucleoside analog, usually administered orally as the diacetyl ester, famciclovir, which acts as a prodrug undergoing rapid first-pass metabolism to release the active compound in vivo. The parent compound has virtually no oral bioavailability, but is supplied as a topical formulation.
Pharmacokinetics
Penciclovir is the active metabolite of the oral product famciclovir. The more favorable results observed with topical penciclovir versus topical acyclovir for the treatment of herpes labialis may be due to the longer intracellular half-life of penciclovir in HSV-infected cells. The activated drug inhibits the viral DNA polymerase. This impairs the ability of the virus to replicate within the cell.
Pharmacokinetics
Oral absorption, penciclovir: 5%
famciclovir: 77%
Cmax famciclovir 250 mg oral: 1.6 mg/L after 0.5–1.5 h
famciclovir 500 mg oral: 3.3 mg/L after 0.5–1.5 h
famciclovir 750 mg oral: 5.1 mg/L after 0.5–1.5 h
Plasma half-life: 2.1–2.7 h
Volume of distribution: c. 1.5 L/kg
Plasma protein binding: <20%
Following absorption famciclovir is converted rapidly by
enzyme-mediated deacetylation and oxidation to penciclovir.
Food does not lead to any significant change in the availability
or elimination.
The pharmacokinetics in elderly subjects are similar to
those seen in younger subjects, although small increases in
AUC and plasma half-lives were seen, consistent with slightly
decreased renal clearance.
Renal excretion is the major route of elimination, 50–60%
of an oral dose being recovered in the urine. After intravenous
infusion, about 70% is excreted unchanged in the urine.
After oral administration of famciclovir, penciclovir accounts
for 82% of urinary drug-related material. The remainder
includes metabolites, of which the largest is the 6-deoxy precursor
of penciclovir. Renal clearance exceeds glomerular filtration,
indicating renal tubular secretion.
Clinical Use
Herpes zoster and genital herpes
Orolabial herpes (topical)
Clinical Use
Penciclovir is approved as a topical formulation for the treatment of herpes labialis. In immunocompetent individuals, penciclovir shortens the duration of lesion presence and pain by approximately half a day when it is initiated within an hour of lesion development and applied every 2 hours during waking hours for 4 days.
Side Effects
In clinical trials the incidence of adverse events after famciclovir, aciclovir and placebo were similar, the most common adverse events being headache and nausea.
Metabolism
Hepatic
References
[1] boyd m r, bacon t h, sutton d, et al. antiherpesvirus activity of 9-(4-hydroxy-3-hydroxy-methylbut-1-yl) guanine (brl 39123) in cell culture. antimicrobial agents and chemotherapy, 1987, 31(8): 1238-1242.
[2] hodge r a, perkins r m. mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl) guanine (brl 39123) against herpes simplex virus in mrc-5 cells. antimicrobial agents and chemotherapy, 1989, 33(2): 223-229.
Properties of Penciclovir
| Melting point: | 275-277°C |
| Boiling point: | 653.4±65.0 °C(Predicted) |
| Density | 1.68±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| solubility | 0.02 M potassium phosphate: soluble2mg/mL |
| form | White solid |
| pka | 14.42±0.10(Predicted) |
| color | White to Off-White |
| λmax | 253nm(H2O)(lit.) |
| Merck | 14,7083 |
| CAS DataBase Reference | 39809-25-1(CAS DataBase Reference) |
| EPA Substance Registry System | 6H-Purin-6-one, 2-amino-1,9-dihydro-9-[4-hydroxy-3-(hydroxymethyl)butyl]- (39809-25-1) |
Safety information for Penciclovir
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for Penciclovir
Penciclovir manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![2-[2-(2-AMINO-9H-PURIN-9-YL)ETHYL]-1,3-PROPANEDIOL](https://img.chemicalbook.in/CAS/GIF/104227-86-3.gif)
You may like
-
 39809-25-1 Penciclovir 98%View Details
39809-25-1 Penciclovir 98%View Details
39809-25-1 -
 Penciclovir 98%View Details
Penciclovir 98%View Details
39809-25-1 -
 Penciclovir 39809-25-1 98%View Details
Penciclovir 39809-25-1 98%View Details
39809-25-1 -
 Penciclovir CAS 39809-25-1View Details
Penciclovir CAS 39809-25-1View Details
39809-25-1 -
 Penciclovir 95.00% CAS 39809-25-1View Details
Penciclovir 95.00% CAS 39809-25-1View Details
39809-25-1 -
 Penciclovir CAS 39809-25-1View Details
Penciclovir CAS 39809-25-1View Details
39809-25-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
