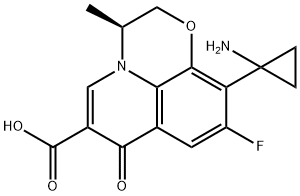
Pazufloxacin
- CAS NO.:127045-41-4
- Empirical Formula: C16H15FN2O4
- Molecular Weight: 318.3
- MDL number: MFCD00865012
- EINECS: 635-036-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-25 23:13:37
What is Pazufloxacin?
Description
Pazufloxacin is a novel quinolone marketed for the treatment of bacterial infections in Japan. This tricyclic fluoro-quinolone can be synthesized in 11 steps from commercially available 2,3,4,5tetrafluorobenzoic acid. The cyclopropyl substituent is first introduced in 6 steps including 4-F-substitution with tert-butylcyanoacetate, decarboxylation, aa alkylation with 1 ,Zdibromoethane, partial nitrile hydrolysis and Hoffmann-rearrangement. The pyridoxazine ring is then introduced in 5 steps including 6-ketoester formation and pryridoxazine annulation. Pazufloxacin displays a broad spectrum activity against Grampositive and Gram-negative bacteria, although it is less active that ciprofloxacin against pneumococci and is not active against ciprofloxacin-resistant isolates. In patients with gonococcal urethritis a high prevalence of fluoroquinolone-resistant N. gonorrhoeae isolates with the Ser-91-to-Phe mutation in GyrA was observed. However, good clinical responses have been seen in clinical trials of patients with urinary tract infections and to a lesser extent with respiratory tract infections. Pazufloxacin is mainly excreted in urine with a short half-life (2-2.5 h). It has a phototoxicity equal to that of ciprofloxacin and its adverse effect profile resembles that of other quinolones.
Originator
Toyama (Japan)
The Uses of Pazufloxacin
Pazufloxacin is a potential antimicrobial and/or antiviral agent.
The Uses of Pazufloxacin
antibactierial
Definition
ChEBI: LSM-5745 is a member of quinolines.
brand name
Pasil, Pazucross
Pharmaceutical Applications
A tricyclic fluoroquinolone, formulated as mesylate and hydrochloride salts for oral or parenteral use or as a methane sulfonate (eye ointment).
It displays good activity in vitro against methicillin
susceptible Staph. aureus (MIC 0.2 mg/L), but is inactive against Str. pyogenes, Str. pneumoniae (MIC ≥4 mg/L) and enterococci. L. pneumophila is inhibited by 0.03 mg/L. Activity against Enterobacteriaceae, fastidious Gram-negative bacilli, Ps. aeruginosa and Acinetobacter spp. is similar to that of ofloxacin. It is weakly active against Sten. maltophilia and Burkholderia cepacia (MIC c. 2 mg/L). Against M. tuberculosis, MICs range from 0.8 to 4 mg/L. It is inactive against anaerobes.
After oral doses of 100 or 400 mg, peak plasma concentrations range from 0.94 mg/L (100 mg) to 4.5 mg/L (400 mg) after <1 h. The apparent elimination half-life is around 2 h. Most of the administered dose is eliminated in urine, about 70% within 24 h. Four metabolites have been reported. In elderly patients, according to the renal function, the peak plasma concentration may be elevated (up to 5.6 mg/L) and significantly delayed (2–6 h). The plasma protein binding ranges from 17% to 28%.
Properties of Pazufloxacin
| Melting point: | 269-271°C |
| Boiling point: | 531.5±50.0 °C(Predicted) |
| alpha | D25 -88.0° (c = 0.5 in 0.05N aq NaOH) |
| Density | 1.56 |
| storage temp. | 2-8°C |
| solubility | Aqueous Base (Slightly, Sonicated), DMSO (Slightly, Heated), Methanol (Slightly, |
| form | neat |
| pka | 5.05±0.40(Predicted) |
| color | Off-White to Pale Yellow |
| CAS DataBase Reference | 127045-41-4(CAS DataBase Reference) |
Safety information for Pazufloxacin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Pazufloxacin
Pazufloxacin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Pazufloxacin CAS 127045-41-4View Details
Pazufloxacin CAS 127045-41-4View Details
127045-41-4 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1