Balofloxacin
Synonym(s):(±)-1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[3-(methylamino)piperidino]-4-oxo-3-quinolinecarboxylic acid
- CAS NO.:127294-70-6
- Empirical Formula: C20H24FN3O4
- Molecular Weight: 389.42
- MDL number: MFCD00864925
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-15 19:19:13
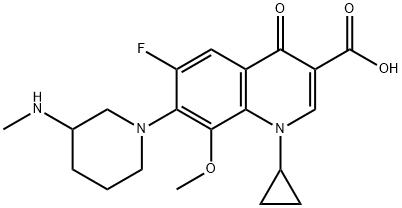
What is Balofloxacin?
Description
Balofloxacin, a novel orally-active fluoroquinolone antibiotic, was introduced in South Korea for the treatment of urinary tract infections (UTI). It can be synthetized by reaction of 3-(methylamino)piperidine with the classical 4-quinolone-3-carboxylic acid template. In vitro antibacterial activity of balofloxacin against gram-positive bacteria (Staphylococcus aureus including methicillin-resistant S. aureus, Staphylococcus epidermis, Streptococcus pneumonia, Streptococcus pyrogenes) was almost equal to that of sparfloxacin or tosufloxacin, in contrast its activity against gram-negative bacteria was 2 times or more lower. In clinical trials, balofloxacin was well tolerated and showed comparable efficacy to ofloxacin in patients with UTls. After oral administration, balofloxacin was well absorbed, and was primarily eliminated unchanged in the urine with an elimination half-life of approximately 8 h. In animal studies, balofloxacin did not exhibit any phototoxicity.
Chemical properties
Crystalline Solid
Originator
Chugai Pharmaceutical (Japan)
The Uses of Balofloxacin
Balofloxacin is quinolone antibiotic, inhibiting the synthesis of bacterial DNA by interference with the enqyme DNA gyrase.
The Uses of Balofloxacin
Fluorinated quinolone antibacterial.
Manufacturing Process
(1) A mixture of ethyl 1-cyclopropyl-6,7,8-trifluoro-1,4-dihydro-4-
oxoquinoline-3-carboxylate (933 mg), 3-acetamidopiperidine (710 mg),
triethylamine (400 mg) and dimethylsulfoxide (10 ml) was heated at 100°C
for 2 hours with stirring. Thereafter the mixture was cooled down and ice
water was added thereto. The resulting mixture was extracted with chloroform
and the chloroform layer was washed with water three times before being
dried over anhydrous sodium sulfate. Removal of the solvent in vacuum
followed by purification by silica gel column chromatography (chloroformethanol)
gave ethyl 7-(3-acetamidopiperidin-1-yl)-1-cyclopropyl-6,8-difluoro-
1,4-dihydro-4-oxo quinoline-3-carboxylate (930 mg). Re-crystallization from
ethanol-ether afforded a colorless crystalline substance (MP: 217°-218°C).
(2) Ethyl 7-(3-acetamidopiperidin-1-yl)-1-cyclopropyl-6,8-difluoro-1,4-dihydro-
4-oxo-quinoline-3-carboxylate obtained from the foregoing step:
(a) (433 mg) of above product was dissolved in 6 N hydrochloric acid (5 ml)
and heated at 100°C. for 2.5 hours with stirring. After the removal of the
solvent in vacuum, methanol was added to the residue and the insoluble
materials were filtered off. Removal of the solvent followed by purification by
silica gel column chromatography (chloroform-methanol) gave hydrochloride
of 7-(3-aminopiperidin-1-yl)-1-cyclopropyl-6,8-difluoro-1,4-dihydro-4-
oxoquinoline-3-carboxylic acid (colorless, crystalline-powder). MP: color
change at about 272°C, decomposition at about 280°C.
(b) A mixture of 7-(3-acetamidopiperidin-1-yl)-1-cyclopropyl-6,8-difluoro-1,4-
dihydro-4-oxoquinoline-3-carboxylic acid (4.05 g), sodium methoxide (2.16 g)
and N,N-dimethylformamide (120 ml) was stirred for 2 hours at 100°-140°C.
The reaction mixture was concentrated in vacuum and water was added to the
residue. The mixture was neutralized with 1 N hydrochloric acid and the
neutralized mixture was then concentrated in vacuum. Purification of the
concentrated mixture by silica gel column chromatography (chloroformmethanol)
gave 7-(3-acetamidopiperidin-1-yl)-1-cyclopropyl-6-fluoro-1,4-
dihydro-8-methoxy-4-oxoquinoline-3-carboxylic acid. MP: 248°-250°C.
(c) 7-(3-Acetamidopiperidin-1-yl)-1-cyclopropyl-6-fluoro-1,4-dihydro-8-
methoxy-4-oxoquinoline-3-carboxylic acid (1.25 g) above obtained was
suspended in 6 N hydrochloric acid (30 ml) and ethanol (5 ml) and heated at
100°C for 3 hours. Then the reaction mixture was concentrated in vacuum
and the residue was purified by silica gel column chromatography
(chloroform:methanol:ammonium hydroxide=100:30:5) to afford 7-(3-
aminopiperidin-1-yl)-1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-4-
oxoquinoline-3-carboxylic acid. MP: 176°-177°C.
brand name
Q-Roxin
Therapeutic Function
Antibacterial
Pharmaceutical Applications
A 6-fluoro-8-methoxy quinolone derivative. It has good antistaphylococcal activity (MIC 0.4–4 mg/L), but is inactive against methicillin-resistant Staph. aureus (MRSA) and quinolone-resistant Staph. aureus; Str. pneumoniae is inhibited by 0.4 mg/L. It has good activity against Enterobacteriaceae, but is inactive against Ps. aeruginosa (MIC 8–16 mg/L). After a 200 mg oral dose a peak level of 1.7 mg/L is reached in 1 h. The apparent elimination halflife is about 8 h, rising to 13 h in elderly subjects. Plasma protein binding is about 16%. It was withdrawn from the market in Japan because of adverse events, but is available in China.
Properties of Balofloxacin
| Melting point: | 137°C |
| Boiling point: | 608.3±55.0 °C(Predicted) |
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | H2O : < 0.1 mg/mL (insoluble)DMSO : 0.67 mg/mL (1.72 mM; Need ultrasonic) |
| form | neat |
| pka | 6.44±0.50(Predicted) |
| form | Solid |
| color | White to off-white |
| BRN | 8362117 |
| CAS DataBase Reference | 127294-70-6(CAS DataBase Reference) |
Safety information for Balofloxacin
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P403:Store in a well-ventilated place. |
Computed Descriptors for Balofloxacin
Balofloxacin manufacturer
Inchem Laboratories Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 127294-70-6 Balofloxacin 98%View Details
127294-70-6 Balofloxacin 98%View Details
127294-70-6 -
 127294-70-6 98%View Details
127294-70-6 98%View Details
127294-70-6 -
 Balofloxacin 98%View Details
Balofloxacin 98%View Details
127294-70-6 -
 Balofloxacin 98% CAS 127294-70-6View Details
Balofloxacin 98% CAS 127294-70-6View Details
127294-70-6 -
 Balofloxacin CAS 127294-70-6View Details
Balofloxacin CAS 127294-70-6View Details
127294-70-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1