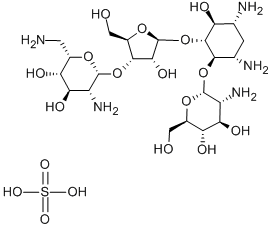
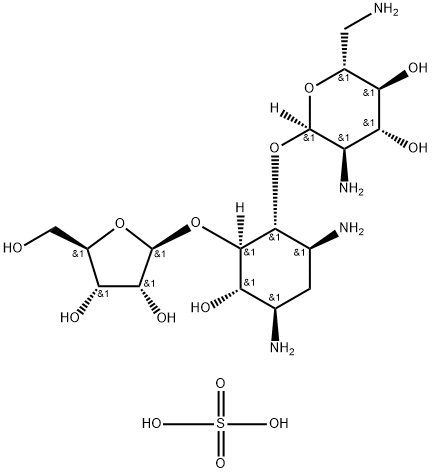
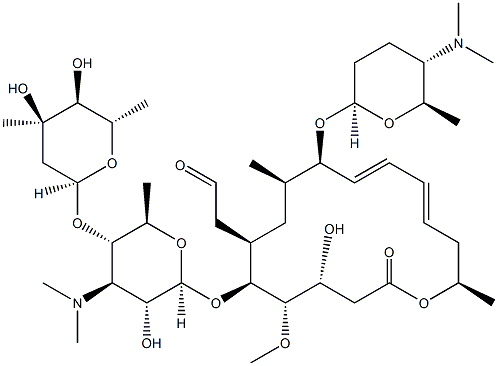
PAROMOMYCIN SULFATE
Synonym(s):Aminosidine sulfate;Neomycin E;Paromomycin Sulfate - CAS 1263-89-4 - Calbiochem;Paromomycin sulfate salt
- CAS NO.:1263-89-4
- Empirical Formula: C23H47N5O18S
- Molecular Weight: 713.71
- MDL number: MFCD00079278
- EINECS: 215-031-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
What is PAROMOMYCIN SULFATE?
Description
Paromomycin was found in the culture broth of Streptomyces rimosus forma paromomycinus by Parke Davis & Co. in 1959. In the same year it was found in the culture broth of S. Crestomyceticus by Farmitalia and named amminosidin. Paromomycin is related structurally to neomycin, but it has a hydroxyl group at the 6 position, whereas neomycin has an amino group. Its antibacterial activity is weaker than that of neomycin, but its toxicity is much less. Paromomycin is used by intramuscular injection for therapy of respiratory, urinary, and surgical infections and by oral administration to treat dysentery and salmonellosis.
Chemical properties
PAROMOMYCIN SULFATE is White to Off-White Solid
The Uses of PAROMOMYCIN SULFATE
PAROMOMYCIN SULFATE is an aminogycoside antibiotic designed to fight intestinal infections such as cryptosporidiosis, amoebiasis, and leishmaniasis. Its antiprotozoal activity makes it an effecive histomonostatic feed addit ive in turkey poults experimentally infected with Histomonas meleagridis.
The Uses of PAROMOMYCIN SULFATE
antibacterial, antiamebic
The Uses of PAROMOMYCIN SULFATE
polymeric nonionic detergent
What are the applications of Application
Paromomycin Sulfate is an aminoglycoside antibiotic
Definition
ChEBI: An aminoglycoside sulfate salt resulting from the treatment of paromomycin with sulfuric acid. A broad-spectrum antibiotic, it is used for the treatment of acute and chronic intestinal protozoal infections, but is not effective for extraintestinal protozoa infections. It is also used as a therapeutic against visceral leishmaniasis.
brand name
Humatin (King); Humatin (Parke- Davis).
General Description
Chemical structure: aminoglycoside
Biochem/physiol Actions
Paromomycin inhibits the initiation and elongation steps of protein synthesis by binding to 16S ribosomal RNA. Paramomycin binds to the A site, which causes defective polypeptide chains to be produced and leads to cell death.
Clinical Use
The isolation of paromomycin (Humatin) was reported in1956 from a fermentation with a Streptomyces sp. (PD04998), a strain said to resemble S. rimosus very closely.The parent organism had been obtained from soil samplescollected in Colombia. Paromomycin, however, moreclosely resembles neomycin and streptomycin in antibioticactivity than it does oxytetracycline, the antibiotic obtainedfrom S. rimosus.
Paromomycin has broad-spectrum antibacterial activityand has been used for the treatment of GI infections causedby Salmonella and Shigella spp., and enteropathogenic E.coli. Currently, however, its use is restricted largely to thetreatment of intestinal amebiasis. Paromomycin is soluble inwater and stable to heat over a wide pH range.
Safety Profile
Poison by intravenous,subcutaneous, and intramuscular routes. Mildly toxic byingestion. Mutation data reported. When heated to decomposition it emits very toxic fumesof SOx and NOx.
Purification Methods
Purify it by dissolving it in H2O (0.5g/10mL) and adding excess EtOH, filter or collect and wash with EtOH, then Et2O by centrifugation, and dry it in vacuo. An aqueous solution is stable at 37o for a week but longer at 0-5o. The free base [7542-37-2] is a white amorphous powder which should be stored under N2 because it is strongly basic and can absorb CO2 from the atmosphere. It is soluble in MeOH (less soluble in EtOH) and has [] D 25 +65o (c 1.5, MeOH). It is an antimicrobial against Gram +ve and Gram –ve bacteria and is antiamoebic. It inhibits initiation and peptide elongation during protein synthesis. [Haskell et al. J Am Chem Soc 8 1 , 3480, 3482 1959, Hichens & Rinehart J Am Chem Soc 85 1547 1963, Beilstein 18 III/IV 7534.]
Properties of PAROMOMYCIN SULFATE
| Melting point: | 145 °C (decomp) |
| alpha | D25 +50.5° (c = 1.5 in water pH 6) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O: 50 mg/mL store stock solution at 2-8°C. Stable at 37°C for 5 days. |
| form | powder |
| color | White to Off-White |
| Water Solubility | Soluble in water |
| Merck | 14,7041 |
| BRN | 5715182 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 1263-89-4 |
| EPA Substance Registry System | Paromomycin sulfate (1263-89-4) |
Safety information for PAROMOMYCIN SULFATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for PAROMOMYCIN SULFATE
| InChIKey | LJRDOKAZOAKLDU-UDXJMMFXSA-N |
PAROMOMYCIN SULFATE manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Paromomycin Sulfate CAS 1263-89-4View Details
Paromomycin Sulfate CAS 1263-89-4View Details
1263-89-4 -
 Paromomycin sulphate salt, ≥98% (TLC) CAS 1263-89-4View Details
Paromomycin sulphate salt, ≥98% (TLC) CAS 1263-89-4View Details
1263-89-4 -
 Paromomycin sulfate 98% (HPLC) CAS 1263-89-4View Details
Paromomycin sulfate 98% (HPLC) CAS 1263-89-4View Details
1263-89-4 -
 Paromomycin sulfate CAS 1263-89-4View Details
Paromomycin sulfate CAS 1263-89-4View Details
1263-89-4 -
 Paromomycin sulfate 95% CAS 1263-89-4View Details
Paromomycin sulfate 95% CAS 1263-89-4View Details
1263-89-4 -
 Paromomycin sulfate salt CAS 1263-89-4View Details
Paromomycin sulfate salt CAS 1263-89-4View Details
1263-89-4 -
 Paromomycin sulfate salt CAS 1263-89-4View Details
Paromomycin sulfate salt CAS 1263-89-4View Details
1263-89-4 -
 Paromomycin sulfate salt CAS 1263-89-4View Details
Paromomycin sulfate salt CAS 1263-89-4View Details
1263-89-4