Paricalcitol
Synonym(s):(1α, 3β, 7E,22E)-19-Nor-9,10-secoergosta-5,7,22-triene-1,3,25-triol
- CAS NO.:131918-61-1
- Empirical Formula: C27H44O3
- Molecular Weight: 416.65
- EINECS: 658-064-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
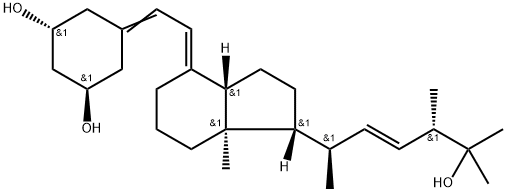
What is Paricalcitol?
Absorption
Well absorbed
Description
Paricalcitol was launched as Zemplar in the US for the prevention and treatment of secondary hyperthyroidism associated with chronic renal failure. Paricalcitol is a synthetic vitamin D2, namely a novel 1-alpha-hydroxy-19-noranalogue in which the ring A exocyclic methylene group, typical of all vitamin D systems, has been replaced by two hydrogen atoms. Paricalcitol is the first vitamin D analogue marketed for this indication. In patients with chronic renal failure, Paricalcitol appreciably reduced levels of parathyroid hormone (PTH) without a significant difference in the incidence rate for hypercalcemia or hyperphosphatemia when compared with placebo.
Description
Paricalcitol is a synthetic 1,25-dihydroxy vitamin D2 analog. As vitamin D deficiency, associated with chronic kidney disease, leads to an increase in parathyroid hormone (secondary hyperparathyroidism), paricalcitol is used in renal patients to block parathyroid hormone overproduction. Vitamin D deficiency is also a risk factor in cancer, cardiovascular disease, hypertension, and diabetes, and paricalcitol may have applications in those contexts as well.
Chemical properties
White Solid
Originator
Abbott (US)
The Uses of Paricalcitol
1,25-Dihydroxy vitamin D2 is a potent agonist of the vitamin D receptor. Paricalcitol is a synthetic 1,25-dihydroxy vitamin D2 analog. As vitamin D deficiency, associated with chronic kidney disease, leads to an increase in parathyroid hormone (secondary hyperparathyroidism), paricalcitol is used in renal patients to block parathyroid hormone overproduction. Vitamin D deficiency is also a risk factor in cancer, cardiovascular disease, hypertension, and diabetes, and paricalcitol may have applications in those contexts as well.
The Uses of Paricalcitol
Synthetic analog of vitamin D. Antihyperparathyroid.
The Uses of Paricalcitol
Antihyperparathyriod
Background
Paricalcitol is a synthetic vitamin D analog. Paricalcitol has been used to reduce parathyroid hormone levels. Paricalcitol is indicated for the prevention and treatment of secondary hyperparathyroidism associated with chronic renal failure.
Indications
For treatment of secondary hyperparathyroidism associated with chronic kidney disease (CKD) Stage 3 and 4
Definition
ChEBI: Paricalcitol is a seco-cholestane and a hydroxy seco-steroid. It has a role as an antiparathyroid drug. It is functionally related to a vitamin D2.
brand name
Zemplar (Abbott).
Pharmacokinetics
Secondary hyperparathyroidism is characterized by an elevation in parathyroid hormone (PTH) associated with inadequate levels of active vitamin D hormone. The source of vitamin D in the body is from synthesis in the skin and from dietary intake. Vitamin D requires two sequential hydroxylations in the liver and the kidney to bind to and to activate the vitamin D receptor (VDR). The endogenous VDR activator, calcitriol [1,25(OH)2 D3], is a hormone that binds to VDRs that are present in the parathyroid gland, intestine, kidney, and bone to maintain parathyroid function and calcium and phosphorus homeostasis, and to VDRs found in many other tissues, including prostate, endothelium and immune cells. VDR activation is essential for the proper formation and maintenance of normal bone. In the diseased kidney, the activation of vitamin D is diminished, resulting in a rise of PTH, subsequently leading to secondary hyperparathyroidism and disturbances in the calcium and phosphorus homeostasis.1 Decreased levels of 1,25(OH)2 D3 have been observed in early stages of chronic kidney disease. The decreased levels of 1,25(OH)2 D3 and resultant elevated PTH levels, both of which often precede abnormalities in serum calcium and phosphorus, affect bone turnover rate and may result in renal osteodystrophy. An in vitro study indicates that paricalcitol is not an inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 or CYP3A at concentrations up to 50 nM (21 ng/mL).
Clinical Use
Vitamin D analogue:
Treatment and prevention of secondary
hyperparathyroidism associated with chronic renal
failure
Metabolism
Metabolized by multiple hepatic and non-hepatic enzymes, including mitochondrial CYP24, as well as CYP3A4 and UGT1A4
Metabolism
Extensively metabolised via hepatic and non-hepatic
pathways to form two relatively inactive metabolites.
After oral administration of 3
H-paricalcitol, only about
2% of the dose was eliminated unchanged in the faeces,
and no parent drug found in the urine.
Approximately
70% of the radioactivity was eliminated in the faeces and
18% was recovered in the urine. Most of the systemic
exposure was from the parent drug.
Properties of Paricalcitol
| Boiling point: | 564.8±50.0 °C(Predicted) |
| Density | 1.121±0.06 g/cm3(Predicted) |
| Flash point: | 14℃ |
| storage temp. | Store at -20°C,unstable in solution, ready to use. |
| solubility | DMSO : 100 mg/mL (240.02 mM; Need ultrasonic)Ethanol : 12.5 mg/mL (30.00 mM; Need ultrasonic)H2O : < 0.1 mg/mL (insoluble) |
| form | neat |
| pka | 14.34±0.40(Predicted) |
| form | Solid |
| color | White to off-white |
| CAS DataBase Reference | 131918-61-1(CAS DataBase Reference) |
Safety information for Paricalcitol
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H330:Acute toxicity,inhalation H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. |
Computed Descriptors for Paricalcitol
| InChIKey | BPKAHTKRCLCHEA-UBFJEZKGSA-N |
| SMILES | C[C@]12CCC/C(=C\C=C3C[C@@H](O)C[C@H](O)C3)/[C@]1([H])CC[C@]2([H])[C@H](C)/C=C/[C@H](C)C(O)(C)C |&1:1,10,13,16,20,22,26,r| |
Paricalcitol manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 131918-61-1 Paricalcitol 98%View Details
131918-61-1 Paricalcitol 98%View Details
131918-61-1 -
 131918-61-1 98%View Details
131918-61-1 98%View Details
131918-61-1 -
 Paricalcitol 98%View Details
Paricalcitol 98%View Details
131918-61-1 -
 Paricalcitol 131918-61-1 98%View Details
Paricalcitol 131918-61-1 98%View Details
131918-61-1 -
 Paricalcitol solution CAS 131918-61-1View Details
Paricalcitol solution CAS 131918-61-1View Details
131918-61-1 -
 Paricalcitol CAS 131918-61-1View Details
Paricalcitol CAS 131918-61-1View Details
131918-61-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
