Palmatine
- CAS NO.:3486-67-7
- Empirical Formula: C21H22NO4+
- Molecular Weight: 352.4
- MDL number: MFCD00221758
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-27 17:31:30
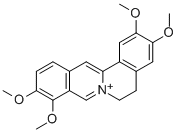
What is Palmatine?
Description
This quaternary alkaloid occurs frequently in the Rhoeadales, being found in Cop tis japonica Mak. (Ranunculaceae); Berberis heteropoda Schrenk; B. vulgaris L. (Berberidaceae); Coscinium blumeatum Miers; Fibrauria chloroleuca Miers;Jatrorrhiza palmata (Lam.) Miers. (Menispermaceae); and Phellodendron amurense Rupr. (Rutcaeae). The base is usually obtained as the iodide dihydrate forming orange-yellow needles from H20, m.p. 241°C (dec.). Other salts that have been prepared include the chloride, green-yellow needles from H20, m.p. 205°C (dec.); nitrate, yellow needles, from H20, m.p. 239°C (dec.); perchlorate, m.p. 262°C (dec.); sulphate, m.p. 250°C; platinichloride, m.p. 236°C and the thiocyanate, m.p. 210°C (dec.). The alkaloid resembles berberine in yielding addition compounds with CHCl3 and Me2CO. On catalytic hydrogenation it gives tetrahydropalmatine, while on oxidation with alkaline KMn04 it furnishes corydaldine and hemipinic acid.
Physical properties
Appearance: yellow needle crystal, odorless, taste very bitter. Solubility: freely solu ble in hot water, sparingly soluble in water, slightly soluble in ethanol and chloroform, and almost insoluble in ether. Melting point: 205 °C. Relative density: 1.2 g/cm3 .
History
In the middle of the last century, Garrison health team of China and Honghe state
hospital interviewed to explore the medicinal use of Huangteng in the screen edge
of ethnic minority areas firstly and found its detoxification and anti-inflammatory
effect of antibacterial. Then, it was promoted to be used in Yunnan province after the
initial clinical validation.
Palmatine on the market is mostly natural, which is mainly obtained from the
extraction of dry rattan of Huangteng, one plant of Menispermaceae. The palmatine
is abundant in the dry rattan of Huangteng with more than 2% palmatine chloride
. It is an important part of technological research to obtain natural palmatine with
high quality and high purity
Honghe state in Yunnan province isolated and extracted palmatine from
Huangteng firstly and then produced needle, tablets, ointment and topical solution,
and other dosage forms for the treatment of various suppurative infections. It is now
believed that the optimum conditions for the extraction of palmatine should be acid water decocting, firstly extracted from the rattan of Huangteng and then adjusted
to pH 9-10, and NaCl salting out, which is obtained by precipitation purifica tion step by step, while ultrasonic can be used as an auxiliary means of extraction.
The growth period of Huangteng is very long so that the extraction of natural
palmatine cannot meet the market demand. Therefore, in order to alleviate the con tradiction of resources, researchers began to explore the method of chemical synthe sis of palmatine and its active derivatives in place of natural palmatine. At present,
the improved synthetic method using berberine as raw material is the one with high
yield, the total yield of which is 54%, and the purity of palmatine is 99%
Indications
Palmatine is included in the Pharmacopoeia of the People’s Republic of China (2015). As an antibacterial drug, palmatine is used to treat upper respiratory tract infections, tonsillitis, enteritis, dysentery, urinary tract infections, surgical and gynecological bacterial infections such as inflammation, and so on. Locally, it can treat conjunctivitis when dropping eyes and Candida albicans infection when used in vaginal topically
Definition
ChEBI: Palmatine is a berberine alkaloid and an organic heterotetracyclic compound. It has a role as a plant metabolite.
Pharmacology
Palmatine is a detoxification agent with a variety of pharmacological effects.
Broad-spectrum antimicrobial antiviral effect: It can inhibit the activity of West
Nile virus NS2B-NS3 protein, the Asian influenza A virus, dengue virus, and yellow
fever virus. Also, it has an inhibitory effect on a variety of Gram-positive and
Gram-negative bacteria, especially on fungi. Palmatine inhibits the proliferation of
the Cordyceps epidermis and other 12 kinds of fungi to different degrees and has
a good effect on shallow or deep infection caused by Candida albicans.
Enhance the ability of leukocyte phagocytosis: 0.3 mL/100 g, once/day, a total of
10 days of intraperitoneal injection can enhance the macrophage phagocytosis func tion. Palmatine has a different degree of prophylactic or therapeutic effect on rat
adjuvant arthritis when orally administered with suspension prepared by 0.5% car boxymethylcellulose for 9 days on dose of 0.5 mg/100 g. Palmatine can partially
counter allergy caused by trichosanthin, and its anti-inflammatory effects may be
related to the immune mechanism. Studies have shown that palmatine can
improve cellular immunity, humoral immunity, and non-specific immune function
Insect resistance: Palmatine has a significant anti-Trichomonas vaginalis activity
in vitro; the anti-vaginal trichomonas activity is equivalent to metronidazole, with
seven different concentrations (20, 10, 5, 2.5, 1.25, 0.625, and 0.3125 μg/mL) and
different times (2–48 h).
Cardiovascular system: Palmatine can protect myocardial infarction, having a
slight excitement on the frog heart and antiarrhythmic effect. It can also lower blood
pressure when injecting intravenously anesthetized rabbits.
In addition, the palmatine also showed an inhibition of central nervous function.
However, the mechanism of pharmacological mechanism of brassinone is still not
clear and need further studies
Clinical Use
Palmatine has an effect of detoxification; therefore, it is used mainly for gynecologi cal inflammation, such as acute and chronic pelvic inflammatory disease, acute and
chronic annex inflammation, cervical erosion, endometritis, mycoplasma vaginitis,
puerperal infection, bacillary dysentery, enteritis, urinary tract infection, surgical
infection, conjunctivitis, respiratory tract infections, and so on.
At present, palmatine has achieved some curative effect in combination therapy,
but the small amount of adverse reactions of palmatine preparations in clinical treat ment cannot be ignored, such as allergic reactions and symptoms of catarr
References
Feist, Sandstede., Arch. Pharm., 256, 1 (1918)
Spath, Quietensky., Ber., 58,2267 (1925)
Haworth, Koepfli, Perkin., J. Chem. Soc., 548 (1927)
Feist, Awe, Etzrodt., Chem. Zentr., I, 2374 (1935)
Spath, Meinhard., Ber., 75,400 (1942)
Properties of Palmatine
| Melting point: | 205°C |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| form | Powder |
| color | Yellow |
| CAS DataBase Reference | 3486-67-7(CAS DataBase Reference) |
Safety information for Palmatine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Palmatine
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Palmatine 98% (HPLC) CAS 3486-67-7View Details
Palmatine 98% (HPLC) CAS 3486-67-7View Details
3486-67-7 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1