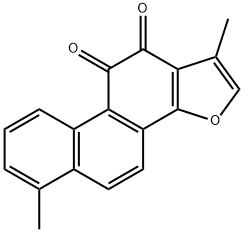
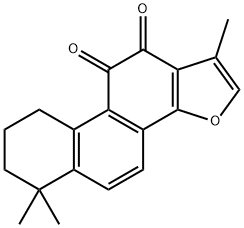
Salvia Root P.E Tanshinone IIA 20%
- Molecular Weight: 0
What is Salvia Root P.E Tanshinone IIA 20%?
Description
Danshen is the dry root and rhizome of Salvia miltiorrhiza Bunge, which belong to
the family of Lamiaceae, genus of Salvia. Danshen was firstly recorded in Shen
Nong’s Classic of Materia Medica. Later, Danshen was recorded in the other ancient
monographs, for example, Wu Pu’s Materia Medica, Miscellaneous Records of
Famous Physicians, and Grand Compendium of Materia Medica. The functions of
Danshen have “eliminating the pathogenic factors in heart and abdomen”, “replenishing
the heart, resuscitation, and relieving mental stress”, “quieting vital fetus and
removing the dead fetus, arresting hemorrhage, mitigating leucorrhea and regulating
the irregular channels of women”, “promoting blood circulation, regulating
pericardium, invigorating qi and nourishing the blood”.
Danshen is widely distributed throughout China and is mainly distributed in
Jiangsu, Anhui, Hebei, Sichuan, Henan, Hubei, Fujian, and Shanxi provinces.
History
Research on the chemical components of Danshen was initiated in the 1930s. Much research on the lipophylic components have been carried out. More than 40 lipophylic compounds have been identified. The research on the hydrosoluble components of Danshen was conducted later. Since the discovery of tanshinol, Chinese scholars isolated a series of hydrosoluble compounds from Danshen. These compounds have the structure of phenolic acid. They are named as salvianolic acids in alphabetic order, salvianolic acids A, B, C, D, E, G, H, tetramethyl salvianolic acid F, isosalvianolic acid C, rosmarinic acid, lithospermic acid, etc. The lipophylic and hydrosoluble compounds have been developed into many preparations for clinical application.
Indications
Danshen preparations are mainly used in treating cardiovascular and cerebrovascular diseases, liver diseases, renal failure, cancer, and so on.
Pharmacology
The study proved that Danshen extracts and their components have many pharmacological actions. It was demonstrated that salvianolic acids have strong antioxidant actions. It has been shown by experiments that salvianolic acids can scavenge oxygen-free radicals and inhibit the peroxide reactions of lipids. Salvianolic acids also have significant inhibiting effects on the aggregation of platelets. Many experiments showed that Danshen can significantly protect the heart and brain against ischemia-reperfusion injury. Danshen also showed obvious protective effects on liver injuries and renal function impairment caused by multiple factors.
Clinical Use
Danshen preparations have been used to treat many diseases, such as cardiovascular disease, stroke, refractory hepatic diseases, pulmonary diseases, hypertension, infectious diseases, cancer, adhesive ileus, etc. Although Danshen has little toxicity, some adverse reactions have been reported. Danshen preparations may lead to adverse cardiovascular effects and should be used with caution.
Safety information for Salvia Root P.E Tanshinone IIA 20%
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6