p-Terphenyl
Synonym(s):1,4-Diphenylbenzene;PT;PTP
- CAS NO.:92-94-4
- Empirical Formula: C18H14
- Molecular Weight: 230.3
- MDL number: MFCD00003061
- EINECS: 202-205-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
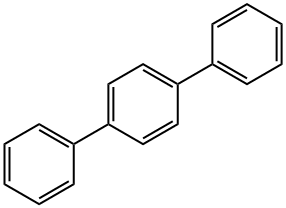
What is p-Terphenyl?
Description
p-Terphenyl(PTP) is an aromatic hydrocarbon isomer, formed by three benzene rings in ortho position. Pure terphenyl is a white crystalline solid, insoluble in water. Though polychlorinated terphenyls were used as heat storage and transfer agents, p-terphenyl is currently under investigation as a material to be used in opto-electronic devices, such as organic LED devices (OLEDs) and currently used in laser dyes and sunscreen lotions. In particle physics it has been used as a wavelength shifter, exploiting its sensitivity to VUV radiation, to read out scintillation light from liquid Xenon. Pterphenyl has also been used as a doping component for liquid scintillators.
So far, p-terphenyl has not been used as main component of particle detectors due to its short Light Attenuation Length. As a matter of fact this property could be exploited when detecting low-energy charged particles in small areas, providing both energy and position measurement.
Chemical properties
a White or light-yellow solid. The melting point is 213°C, boiling point is 383°C, 250°C (6kPa), flash point is 207°C, and relative density is 1.234 (0°C). Soluble in hot benzene, slightly soluble in ether and carbon disulfide, extremely insoluble in ethanol and acetic acid. 427℃ sublimation. p-Terphenyl can be separated from the by-products of biphenyl production.
The Uses of p-Terphenyl
p-Terphenyl is a specialty material, and may be used in ionized radiation detectors, nonpolar laser dye, and single molecule optical probe of scanning near-field microscopy.
Definition
ChEBI: p-Terphenyl is a para-terphenyl that consists of benzene attached to two phenyl units at positions 1 and 4 respectively.
What are the applications of Application
LCD Electronics: P-Terphenyl is one of the basic intermediates for preparing diphenyl LCD materials at present.
Organic synthesis: P-Terphenyl is the basic raw material for synthesizing antifungal cyclic peptide (4-carboxyl P–Terphenyl CTP) and diphenyl polyamide material 4, 4-(DCTP).
Fine chemical engineering: under radiation, P-Terphenyl will generate fluorescence, and thus it can be used as an organic scintillation reagent. It is used as an illuminant in scintillation counters and as a synthetic intermediate for laser dyes.
p-Terphenyl-Sensitized Photoreduction of CO2 with Cobalt and Iron Porphyrins. Interaction between CO and Reduced Metalloporphyrins
Definition
Most of the natural terphenyls are p-terphenyls which consist of a C-18 tricyclic or polycyclic C-18 aromatic skeleton. The chemical investigation of p-terphenyls as one class of the pigments of mushrooms was traced back to 1877. So far, most natural p-terphenyl derivatives have been isolated from basidiomycete macrofungi, while few of them have been reported from endolithic fungi, actinomycetes, and moss. These types of compounds were found to have a broad spectrum of biological effects including immunosuppressive, antioxidative, neuroprotective, antimicrobial, and cytotoxic activities. The fact that some popular edible mushrooms such as Thelephora aurantiotincta, T. ganbajun, and Sarcodon leucopus are rich in p-terphenyls supports the usage of this class of natural products as food supplements to improve human health[1-2].
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 30, p. 802, 1982 DOI: 10.1248/cpb.30.802
The Journal of Organic Chemistry, 50, p. 3104, 1985 DOI: 10.1021/jo00217a018
General Description
White or light-yellow needles or leaves. Mp: 212-213°C; bp 376°C. Density: 1.23 g cm-3. Insoluble in water. Soluble in hot benzene. Very soluble in hot ethyl alcohol. Usually shipped as a solid mixture with its isomers o-terphenyl and m-terphenyl that is used as a heat-transfer fluid.
Reactivity Profile
p-Terphenyl is non-flammable but combustible (flash point: 410°C). Extremely stable thermally. Incompatible with strong oxidizing agents but not very reactive at room conditions.
Hazard
Toxic by ingestion and inhalation. Eye and upper respiratory tract irritant.
Safety Profile
Moderately toxic by ingestion. Combustible when exposed to heat or flame. To fight fire, use water, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
Crystallise p-terphenyl from nitrobenzene or trichlorobenzene. It is also purified by chromatography on alumina in a darkened room, using pet ether, and then crystallising from pet ether (b 40-60o) or pet ether/*benzene. It is a fluorophore for scintillation counting and has ex 286nm : em 343nm in DMF, and max 277nm (log 4.50). [Beilstein 5 IV 2483.]
Structure and conformation
The most prominent character in the structure of p-terphenyl is that they possess an aromatic or a para quinone group in the central ring, which, in nearly all cases, carries additional oxygen functions as well. The structural diversity resulted from the variation of the ring B and the connections between the rings, and to the main skeleton, one or more oxygen functions like hydroxy group, methoxy group or ester group at C-3′, C-4′, C-4′′, or/and C-5′′ were attached. The UV spectrum of p-terphenyls showed absorption maxima at 260–300 nm and more than 300 nm indicating the presence of conjugated or aromatic systems. Their IR spectrum mostly has absorption bands of phenolic hydroxyl (3200–3500 cm-1) and benzene groups (about 1600 and about 1500 cm-1)[1].
References
[1] Zhou, Guoliang , et al. "Structural diversity and biological activity of natural p-terphenyls." Journal of Asian Natural Products Research4.1(2022):12.
[2] Takahashi, S , et al. "Total Synthesis of Kehokorins A–E, Cytotoxic p-Terphenyls."Journal of Organic Chemistry82.6(2017):3159.
Properties of p-Terphenyl
| Melting point: | 212-213 °C(lit.) |
| Boiling point: | 389 °C(lit.) |
| Density | 1.23 |
| vapor density | 7.95 (vs air) |
| refractive index | 1.5500 (estimate) |
| Flash point: | 207 °C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), DMSO (Slightly, Heated, Sonicated) |
| form | Crystals or Crystalline Powder |
| color | White to slightly beige |
| Water Solubility | practically insoluble |
| λmax | 276nm(Cyclohexane)(lit.) |
| BRN | 1908447 |
| Exposure limits | NIOSH: IDLH 500 mg/m3; Ceiling 0.5 ppm(5 mg/m3) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 92-94-4(CAS DataBase Reference) |
| NIST Chemistry Reference | p-Terphenyl(92-94-4) |
| EPA Substance Registry System | p-Terphenyl (92-94-4) |
Safety information for p-Terphenyl
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. P501:Dispose of contents/container to..… |
Computed Descriptors for p-Terphenyl
| InChIKey | XJKSTNDFUHDPQJ-UHFFFAOYSA-N |
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
You may like
-
 p-Terphenyl CAS 92-94-4View Details
p-Terphenyl CAS 92-94-4View Details
92-94-4 -
 p-Terphenyl (purified by sublimation) CAS 92-94-4View Details
p-Terphenyl (purified by sublimation) CAS 92-94-4View Details
92-94-4 -
 P-Terphenyl 98% CAS 92-94-4View Details
P-Terphenyl 98% CAS 92-94-4View Details
92-94-4 -
 p-Terphenyl CAS 92-94-4View Details
p-Terphenyl CAS 92-94-4View Details
92-94-4 -
 p-Terphenyl CAS 92-94-4View Details
p-Terphenyl CAS 92-94-4View Details
92-94-4 -
 1,4-Diphenylbenzene 98%View Details
1,4-Diphenylbenzene 98%View Details -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3
