4-Nitrobenzoic acid
Synonym(s):4-Nitrobenzoic acid;Benzocaine impurity E (PhEur)
- CAS NO.:62-23-7
- Empirical Formula: C7H5NO4
- Molecular Weight: 167.12
- MDL number: MFCD00007352
- EINECS: 200-526-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-14 10:02:33
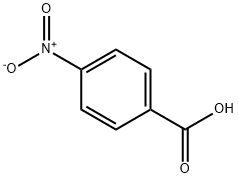
What is 4-Nitrobenzoic acid?
Chemical properties
light yellow crystalline powder
The Uses of 4-Nitrobenzoic acid
4-Nitrobenzoic Acid is used in the synthesis of anti-Trypanosoma cruzi agents in the treatment of chagas disease.
Definition
ChEBI: A nitrobenzoic acid having the nitro group at the 4-position.
Production Methods
p-Nitrobenzoic acid (4-Nitrobenzoic acid) is produced commercially by the oxidation of 4-nitrotoluene with molecular oxygen. Oxidation with 15 % nitric acid at 175 ℃ produces the acid in 88.5 % yield. An interesting method involves the nitration and subsequent oxidation of polystyrene. This method uses the steric hindrance of the polymer chain to improve the para to ortho ratio of the product.
Synthesis Reference(s)
Tetrahedron Letters, 26, p. 2387, 1985 DOI: 10.1016/S0040-4039(00)94834-2
Journal of the American Chemical Society, 72, p. 5012, 1950 DOI: 10.1021/ja01167a051
Air & Water Reactions
Insoluble in water.
Reactivity Profile
p-Nitrobenzoic acid is incompatible with strong oxidizers. p-Nitrobenzoic acid is also incompatible with strong bases (potassium hydroxide). p-Nitrobenzoic acid may react with cyanides.
Fire Hazard
p-Nitrobenzoic acid is combustible.
Toxicology
Among various nitroaromatics, 4-nitrobenzoic acid (4-NBA) is one of the simplest and basic derivates of the explosives. Moreover, 4-NBA harms human health and causes severe water and atmosphere pollution. 4-Nitrobenzoic acid is stable, toxic, and non-biodegradable. US/Zn0 was found to degrade more than 85% of 4-nitrobenzoic acid in 30 min. There was an apparent synergistic effect between ultrasound and Zn[1-2].
Toxicity
As 4-nitrobenzoic acid is a metabolite of 4-nitrotoluene, which also induces tumours of the clitoral gland as well as tumours in other organs, 4-nitrobenzoic acid is classified in analogy to 4-nitrotoluene in Carcinogen Category 3?B. 4-Nitrobenzoic acid has a weak mutagenic potency in vitro. It did not induce micronuclei in vivo or in vitro. There is no suitable data in humans to derive an MAK value. In rats, a 15-month and 2-year study resulted in a NOAEL of 60 mg/ kg body weight and day for delayed body weight gain in the females. Toxicokinetic scaling results in 147 mg/m3 concentration in workplace air. The LOAEC for local effects on the olfactory epithelium of rats in a 14-day study is 150 mg/m3, the NOAEC 20 mg/m3.
Purification Methods
Purify it as for 3-nitrobenzoic acid above. The amide has m 201.6o (from H2O). [Beilstein 9 III 1537, 9 IV 1072.]
References
[1] Shan Chong. “Ultrasound/Zn0 for aqueous 4-nitrobenzoic acid degradation.” Desalination and Water Treatment 322 1 (2016): 24990–24998.
[2] Xue-Huan Xin . “A luminescent barium-based metal-organic framework: Synthesis, structure and efficient detection of 4-nitrobenzoic acid.” Inorganic Chemistry Communications 97 (2018): Pages 129-133.
Properties of 4-Nitrobenzoic acid
| Melting point: | 237-240 °C(lit.) |
| Boiling point: | 295.67°C (rough estimate) |
| Density | 1.61 |
| refractive index | 1.6280 (estimate) |
| Flash point: | 237 °C |
| storage temp. | Store below +30°C. |
| solubility | 0.42g/l |
| form | Crystalline Powder |
| pka | 3.41(at 25℃) |
| color | Light yellow |
| PH | 3.1 (0.42g/l, H2O, 20℃)(saturated solution) |
| Water Solubility | <0.1 g/100 mL at 26 ºC |
| Merck | 14,6589 |
| BRN | 973593 |
| Specific Activity | 50-60 mCi/mmol |
| Concentration | 0.1 mCi/ml |
| Solvent | Ethanol |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, cyanides, strong bases. |
| CAS DataBase Reference | 62-23-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoic acid, 4-nitro-(62-23-7) |
| EPA Substance Registry System | p-Nitrobenzoic acid (62-23-7) |
Safety information for 4-Nitrobenzoic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity H351:Carcinogenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 4-Nitrobenzoic acid
| InChIKey | OTLNPYWUJOZPPA-UHFFFAOYSA-N |
4-Nitrobenzoic acid manufacturer
BTC pharm India
CEFA CILINAS BIOTICS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 4-Nitrobenzoic acid 98%View Details
4-Nitrobenzoic acid 98%View Details -
 4-Nitrobenzoic acid 98%View Details
4-Nitrobenzoic acid 98%View Details -
 Powder 4 Nitro Benzoic Acid, Purity: 99%View Details
Powder 4 Nitro Benzoic Acid, Purity: 99%View Details
62-23-7 -
 Pharma Grade Para Nitrobenzoic Acid Chemical Powder, Purity: 99%View Details
Pharma Grade Para Nitrobenzoic Acid Chemical Powder, Purity: 99%View Details
62-23-7 -
 Powder Para Nitro Benzoic Acid, 25 KgView Details
Powder Para Nitro Benzoic Acid, 25 KgView Details
62-23-7 -
 Para Nitro Benzoic AcidView Details
Para Nitro Benzoic AcidView Details
62-23-7 -
 Industrial Grade 4 Nitrobenzoic AcidView Details
Industrial Grade 4 Nitrobenzoic AcidView Details
62-23-7 -
 INDUSTRIAL Para Nitro Benzoic Acid, For Pharma, Purity: 99.0% MINView Details
INDUSTRIAL Para Nitro Benzoic Acid, For Pharma, Purity: 99.0% MINView Details
62-23-7
