3-Diethylaminophenol
- CAS NO.:91-68-9
- Empirical Formula: C10H15NO
- Molecular Weight: 165.23
- MDL number: MFCD00002265
- EINECS: 202-090-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:46
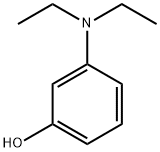
What is 3-Diethylaminophenol?
Chemical properties
Grey-brown to rose or red flakes or granules
The Uses of 3-Diethylaminophenol
3-Diethylaminophenol was used as starting reagent for the synthesis of silyloxyaniline. It was used in the synthesis of Nile Red and its hydroxy-derivative.
The Uses of 3-Diethylaminophenol
3-Diethylaminophenol is used as a reagent in the synthesis of coumarin- and rhodamine-fused deep red fluorescent dyes.
General Description
Black solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
An amine and phenol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Phenols do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid.
Hazard
See phenol.
Health Hazard
ACUTE/CHRONIC HAZARDS: 3-Diethylaminophenol may cause irritation on contact.
Fire Hazard
Flash point data are not available for 3-Diethylaminophenol, but 3-Diethylaminophenol is probably combustible.
Flammability and Explosibility
Non flammable
Properties of 3-Diethylaminophenol
| Melting point: | 69-72 °C (lit.) |
| Boiling point: | 170 °C/15 mmHg (lit.) |
| Density | 1.0203 (rough estimate) |
| vapor pressure | 0.345Pa at 25℃ |
| refractive index | 1.4820 (estimate) |
| Flash point: | 141 °C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | 4.087g/L in organic solvents at 20 ℃ |
| form | Flakes or Granules |
| pka | 10.08±0.10(Predicted) |
| color | Gray-brown to rose or red |
| Water Solubility | <0.1 g/100 mL at 20.5 ºC |
| BRN | 908212 |
| CAS DataBase Reference | 91-68-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 3-(diethylamino)-(91-68-9) |
| EPA Substance Registry System | m-(Diethylamino)phenol (91-68-9) |
Safety information for 3-Diethylaminophenol
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for 3-Diethylaminophenol
3-Diethylaminophenol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 91-68-9 Diethyl Meta Amino Phenol (DEMAP) 98%View Details
91-68-9 Diethyl Meta Amino Phenol (DEMAP) 98%View Details
91-68-9 -
 91-68-9 98%View Details
91-68-9 98%View Details
91-68-9 -
 3-Diethylaminophenol 99%View Details
3-Diethylaminophenol 99%View Details
91-68-9 -
 91-68-9 3-Diethylaminophenol 99%View Details
91-68-9 3-Diethylaminophenol 99%View Details
91-68-9 -
 3-Diethylaminophenol, 99% CAS 91-68-9View Details
3-Diethylaminophenol, 99% CAS 91-68-9View Details
91-68-9 -
 N,N-Diethyl-3-aminophenol CAS 91-68-9View Details
N,N-Diethyl-3-aminophenol CAS 91-68-9View Details
91-68-9 -
 3-DI ETHYL AMINO PHENOL (DEMAP)View Details
3-DI ETHYL AMINO PHENOL (DEMAP)View Details
91-68-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
