(-)-EBURNAMONINE
- CAS NO.:4880-88-0
- Empirical Formula: C19H22N2O
- Molecular Weight: 294.39
- MDL number: MFCD00064309
- EINECS: 225-490-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
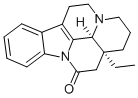
What is (-)-EBURNAMONINE?
Chemical properties
white to slightly yellow crystalline powder
Originator
Euburnamonine ,Shanghai Lansheng Corporation
The Uses of (-)-EBURNAMONINE
(-)-Eburnamonine shows protective effects on mice from the lethal consequences of hypoxia.
The Uses of (-)-EBURNAMONINE
Cerebral vasodilatator
What are the applications of Application
(?)-Eburnamonine is a vasodilator that also acts as a cerebral metabolic stimulant
Definition
ChEBI: Eburnamonine is an alkaloid.
Manufacturing Process
200 g (0.087 moles) of cis-1-ethyl-1-(2'-hydroxy-2'-carboxyethyl)-
1,2,3,4,6,7,12,12b-octahydro-indolo[2,3-a]quinolizine were suspended in 100
ml of xylene. 12 g of supported silver carbonate (a silver carbonate-Celite
reagent, containing 50% of silver carbonate) were added to the suspension,
and the system was boiled for 8 hours under a nitrogen atmosphere, with
constant stirring. Thereafter the hot solution was filtered, washed with 3 x 30
ml to 5% sodium carbonate solution, dried over magnesium sulfate, and
evaporated under reduced pressure. 1.05 g of a solid residue, which was a
mixture of racemic vincamone and racemic vincanol, was obtained. 40 ml of
ethyl ether were added to the mixture, and the insoluble crystals were filtered
off. The crystals were recrystallized from methanol, to yield 0.5 g of (+/-)-
eburnamonine (vincamone). Yield: 29.5%. MP: 201°-202°C. The value was
identical with the literature value (J. Mokry et aI.: CoII. Czech, Chem. Comm.
28, 1309, 1963). The etheral mother liquor was processed by preparative
layer chromatography (adsorbent: Kieselgel PF 254+366 , developing agent: a
14:2 mixture of benzene and methanol). The obtained substance was
recrystallized from ether to yield 0.2 g (16%) of (+/-)-vincanol (eburnamine).
MP: 163-164°C, under decomposition. The IR spectrum of the obtained
substance was identical with that of the authentic sample.
Resolution of (+/-)-vincamone:
10.0 g (0.0342 moles) of (+/-)-vincamone and 12.2 g (0.0342 moles) of (-)-
dibenzoyl tartaric acid were dissolved in 150 ml of dichloromethane, the
solution was seeded with crystalline (-)-vincamone-(-)-dibenzoyl-tartarate,
and the mixture was left to stand. The separated crystals were filtered off,
dissolved in dimethylformamide, and the pH of the solution was adjusted to 9 with aqueous ammonia. The separated substance was filtered off, washed with
water, and dried. This way 4.2 g (84%) of (-)-vincamone were obtained; MP:
-176°C; α D 20 = -96°. The mother liquor of resolution was evaporated and (+)-
vincamone was separeted as described above. The obtained substance had
MP: 173°-176°C.; α D 20 = +96°.
Therapeutic Function
Cerebrotonic
Properties of (-)-EBURNAMONINE
| Melting point: | 174-177 °C(lit.) |
| Boiling point: | 436.16°C (rough estimate) |
| alpha | D25 -102° (chloroform) (Clauder); D -100° (c = 0.783 in chloroform) (Cartier) |
| Density | 1.34 |
| refractive index | 1.5600 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly, Heated) |
| form | White to slightly yellow crystalline powder. |
| pka | 8.13±0.40(Predicted) |
| color | White |
| Merck | 13,3520 |
| CAS DataBase Reference | 4880-88-0(CAS DataBase Reference) |
Safety information for (-)-EBURNAMONINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for (-)-EBURNAMONINE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![2-[1-(Dimethylamino)ethyl]indole](https://img.chemicalbook.in/CAS/GIF/96286-10-1.gif)
![2-[1-(Methylamino)ethyl]indole](https://img.chemicalbook.in/CAS/GIF/96286-08-7.gif)
![2,3,4,9-Tetrahydro-1-isopropyl-1H-pyrido[3,4-b]indole](https://img.chemicalbook.in/CAS/GIF/6650-04-0.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1