Oxatomide
Synonym(s):1-[3-[4-(Diphenylmethyl)-1-piperazinyl]propyl]-1,3-dihydro-2H-benzimidazol-2-one
- CAS NO.:60607-34-3
- Empirical Formula: C27H30N4O
- Molecular Weight: 426.55
- MDL number: MFCD00211147
- EINECS: 262-320-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-19 15:37:51
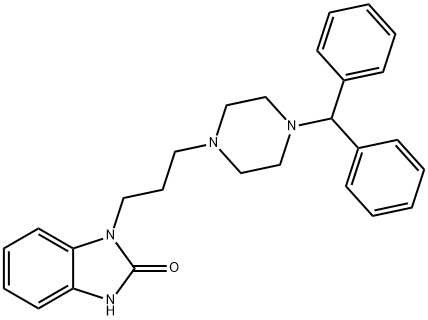
What is Oxatomide?
Chemical properties
White Powder
Originator
Tinset,Janssen,W. Germany ,1981
The Uses of Oxatomide
Orally active anti-allergic agent; related structurally to cinnarizine and having a novel biphasic mode of action
The Uses of Oxatomide
Inhibits the release and actions of leukotrienes
The Uses of Oxatomide
Orally active anti-allergic agent; related structurally to cinnarizine and having a novel biphasic mode of action.
Background
Oxatomide has been used in trials studying the treatment of Muscular Dystrophy, Duchenne.
What are the applications of Application
Oxatomide is an H1-histamine receptor antagonist
Definition
ChEBI: Oxatomide is a member of the class of benzimidazoles that is 1,3-dihydro-2H-benzimidazol-2-one substituted by a 3-[4-(diphenylmethyl)piperazin-1-yl]propyl group at position 1. It is an anti-allergic drug. It has a role as a geroprotector, a H1-receptor antagonist, an anti-allergic agent, an anti-inflammatory agent and a serotonergic antagonist. It is a N-alkylpiperazine, a member of benzimidazoles and a diarylmethane.
Manufacturing Process
A mixture of 53 parts of 1-(3chloropropyl)-2H-benzimidazol-2one, 5 parts of 1-(diphenylmethyl)piperazine, 6.4 parts of sodium bicarbonate and 200 parts of 4-methyl-2-pentanone is stirred and refluxed overnight with waterseparator. After cooling, water is added and the layers are separated. The 4methyl-2pentanone phase is dried, filtered and evaporated. The residue is purified by column-chromatography over silica gel using a mixture of trichloromethane and 5% of methanol as eluent. The pure fractions are collected and the eluent is evaporated. The oily residue is crystallized from a mixture of 2,2'-oxybispropane and a small amount of 2-propanol. The product is filtered off and dried, yielding 1-[3-[4-(diphenylmethyl)-1piperazinyl]propyl]-2H-benzimidazole-2-one; melting point 153.6°C.
Therapeutic Function
Antiallergic
Safety Profile
Poison by ingestion, intraperitoneal, and intravenous routes. An experimental teratogen. Experimental reproductive effects. Used to treat allergies and asthma. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
Oxatomide is synthesized by alkylation of 1-diphenylmethylpiperazine with 1-(3-chloropropyl)-1,3-dihydro-2Hbenzimidazol- 2-one in the presence of Na2CO3 . In addition to its histamine H1 receptor antagonistic effect, oxatomide may have mast-cell stabilizing activities .
Metabolism
Not Available
Properties of Oxatomide
| Melting point: | 153.60C |
| Boiling point: | 541.98°C (rough estimate) |
| Density | 1.1239 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Refrigerator |
| solubility | DMSO: soluble |
| form | White solid. |
| pka | 12.20±0.30(Predicted) |
| color | white |
| CAS DataBase Reference | 60607-34-3(CAS DataBase Reference) |
Safety information for Oxatomide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Oxatomide
Oxatomide manufacturer
Fleming Laboratories Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 60607-34-3 Oxatomide 99%View Details
60607-34-3 Oxatomide 99%View Details
60607-34-3 -
 60607-34-3 98%View Details
60607-34-3 98%View Details
60607-34-3 -
 Oxatomide CAS 60607-34-3View Details
Oxatomide CAS 60607-34-3View Details
60607-34-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1