Ornipressin
- CAS NO.:3397-23-7
- Empirical Formula: C45H63N13O12S2
- Molecular Weight: 1042.19
- MDL number: MFCD16659865
- EINECS: 222-253-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-26 10:35:11
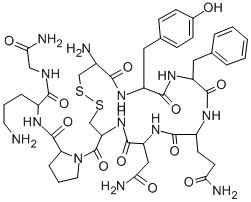
What is Ornipressin?
Originator
POR-8 ,Sandoz,W. Germany ,1977
The Uses of Ornipressin
Ornipressin is an analogue of Vasopressin. Ornipressin is used as a vasoconstrictor, haemostatic and renal agent.
Definition
ChEBI: Ornipressin is an oligopeptide.
Manufacturing Process
(a)N-α-carbobenzoxy-N-δ-p-toluenesulfonyl-L-ornithyl-glycine ethyl ester: 104 g of N-α-carbobenzoxy-N-δ-p-toluenesulfonyl-L-ornithine and 27 g of glycine ethyl ester are dissolved in 450 cc of acetonitrile, the mixture is cooled at 0°C, 51 g of dicyclohexyl carbodiimide are added and the mixture is shaken at room temperature for 4 hours. Precipitated dicyclohexyl urea is filtered off and washed with acetonitrile. The whole filtrate is evaporated in a vacuum. The residue crystallizes after the addition of petroleum ether. After recrystallization from n-propanol, 93 g of N-α-carbobenzoxy-N-δ-toluenesulfonyl-L-ornithylglycine ethyl ester are obtained; melting point 136°C; [α]D22 = -6.5° (96% ethanol).
(b) N-carbobenzoxy-L-prolyl-N-δ-p-toluenesulfonyl-L-ornithyl-glycinamide: 90 g of N-α-carbobenzoxy-N-δ-p-toluenesulfonyl-L-ornithyl-glycine ethyl ester are dissolved in 800 cc of anhydrous acetic acid which has been saturated with hydrogen bromide. The mixture is left to stand for one hour at 20°C, evaporated in a vacuum at a temperature below 40°C and the residue washed carefully with diethyl ether. The residue is dissolved in 500 cc of acetonitrile, 25 cc of triethylamine and 43 g of N-carbobenzoxy-L-proline are added, cooling is effected at 0°C, 355 g of dicyclohexyl carbodiimide are then added and the mixture shaken overnight at 20°C. After filtering off dicyclohexyl urea, the filtrate is evaporated in a vacuum at 30°C, the residue dissolved in ethyl acetate and this solution is washed with dilute sulfuric acid and aqueous
ammonia. After drying over sodium sulfate, the ethyl acetate is removed by evaporation in a vacuum and the residue dissolved in 1 liter of absolute ethanol. The solution is cooled at 0°C, saturated with ammonia and left to stand overnight at 20°C. After evaporating in a vacuum at 30°C, the residue is recrystallized from dimethylformamide/ethyl acetate. 58 g of Ncarbobenzoxy-L-prolyl-N-δ-p-toluenesulfonyl-L-ornithyl-glycinamide are obtained: melting point 122°C (with decomposition).
(c) N-carbobenzoxy-L-glutaminyl-L-asparaginyl-S-benzyl-L-cystsinyl-L-prolyl-Nδ-p-toluenesulfonyl-L-ornithyl-glycinamide: 100 g of N-carbobenzoxy-L-prolylN-δ-p-toluenesulfonyl-L-ornithyl-glycinamide are dissolved in 500 cc of anhydrous acetic acid which has been saturated with hydrogen bromide, the solution is left to stand for one hour at 20°C and is evaporated in a vacuum at a temperature below 40°C. The residue is carefully washed with diethyl ether and then added to a solution of 100 g of N-carbobenzoxy-L-glutaminyl-Lasparaginyl-S-benzyl-L-cysteinyl-azide and 26 cc of triethylamine in 1,000 cc of dimethylformamide. The mixture is left to stand overnight at 20°C, 3,000 cc of ethyl acetate are added thereto, the precipitate is filtered off and washing is effected with ethyl acetate. 105 g of N-carbo-benzoxy-Lglutaminyl-L-asparaginyl-S-benzyl-L-cysteinyl-L-prolyl-N-δ-p-toluenesulfonyl-Lornithyl-glycinamide are obtained; melting point 193°C; [α]D20 = 38.5°(dimethylformamide).
(d) N-carbobenzoxy-S-benzyl-L-cysteinyl-L-tyrosyl-L-phenyl-alanyl-Lglutaminyl-L-asparaginyl-S-benzyl-L-cysteinyl-L-prolyl-N-δ-p-toluenesulfonyl-Lornithyl-glycinamide: 50 g N-carbobenzoxy-L-glutaminyl-L-asparaginyl-Sbenzyl-L-cysteinyl-L-prolyl-N-δ-p-toluenesulfonyl-L-ornithyl-glycinamide are dissolved in 250 cc of anhydrous acetic acid which has been saturated with hydrogen bromide and the solution is left to stand for one hour at 20°C. After evaporating the solvent in a vacuum at a temperature below 40°C, the residue is carefully washed with diethyl ether and a solution of 31.5 g of Ncarbobenzoxy-S-benzyl-L-cysteinyl-L-tyrosyl-L-phenylalanine-azide and 7.5 cc of triethylamine in 250 cc of dimethylformamide is added thereto. The mixture is left to stand for 2 days at 20°C. 1,000 cc of ethyl acetate are subsequently added and the precipitate is washed with ethyl acetate. After drying in a vacuum at 30°C. the product is washed with warm methanol. 45 g of Ncarbobenzoxy-S-benzyl-L-cysteinyl-L-tyrosyl-L-phenylalanyl-L-glutaminyl-Lasparaginyl-S-benzyl-L-cysteinyl-L-prolyl-N-δ-p-toluenesulfonyl-L-ornithylglycinamide are obtained; melting point 224°C.
(e) L-cysteinyl-L-tyrosyl-L-phenylalanyl-L-glutaminyl-L-asparaginyl-L-cysteinylL-prolyl-L-ornirhyl-glycinamide: The necessary amount of sodium or potassium metal is added to a solution of 5 g of N-carbobenzoxy-S-benzyl-L-cysteinyl-Ltyrosyl-L-phenylalanyl-L-glutaminyl-L-asparaginyl-S-benzyl-L-cysteinyl-L-prolylN-δ-p-toluenesulfonyl-L-ornithyl-glycinamide in 1,200 cc of dry liquid ammonia, while stirring at the boiling temperature of the solution, to give a stable blue coloration. After the addition of 3 g of ammonium chloride, the solution is evaporated to dryness. The residue contains L-cysteinyl-L-tyrosyl-Lphenyl-alanyl-L-glutaminyl-L-asparaginyl-L-cysteinyl-L-prolyl-L-ornithylglycinamide.
Therapeutic Function
Vasoconstrictor
Properties of Ornipressin
| Boiling point: | 1616.2±65.0 °C(Predicted) |
| Density | 1.320±0.06 g/cm3(Predicted) |
| solubility | DMSO (Very Slightly, Sonicated), Methanol (Very Slightly, Sonicated) |
| form | Solid |
| pka | 9.90±0.15(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 3397-23-7(CAS DataBase Reference) |
Safety information for Ornipressin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ornipressin
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1