Olivetol
Synonym(s):1,3-Dihydroxy-5-pentylbenzene;3,5-Dihydroxyamylbenzene;5-Pentylresorcinol
- CAS NO.:500-66-3
- Empirical Formula: C11H16O2
- Molecular Weight: 180.24
- MDL number: MFCD00002293
- EINECS: 207-908-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
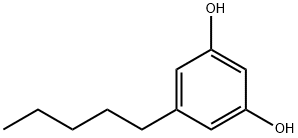
What is Olivetol?
Physical properties
light purple to brown crystalline mass.
Chemical properties
Olivetol is an off-white crystals or olive to light purple waxy solid. Forms monohydrate (melting point: 102-106°F). Olivetol is a member of the class of resorcinols that is resorcinol in which the hydrogen at position 5 is replaced by a pentyl group. It has a role as a lichen metabolite.
The Uses of Olivetol
Olivetol was used as a template molecule in the synthesis of molecularly imprinted polymer (MIP). It was also used as an inhibitor of (S)-mephenytoin 4'-hydroxylase activity of recombinant CYP2C19.
What are the applications of Application
Olivetol is a precursor in various syntheses of tetrahydrocannabinol.
Definition
ChEBI: Olivetol is a member of the class of resorcinols that is resorcinol in which the hydrogen at position 5 is replaced by a pentyl group. It has a role as a lichen metabolite.
Preparation
olivetol can also be formed through hydrolysis of intermediate polyketide CoAs or spontaneous cyclization.
Synthesis Reference(s)
The Journal of Organic Chemistry, 42, p. 3456, 1977 DOI: 10.1021/jo00441a036
General Description
Olivetol appears as off-white crystals or olive to light purple waxy solid. Forms monohydrate (melting point: 102-106°F). (NTP, 1992)
Air & Water Reactions
Sensitive to air. Insoluble in water.
Reactivity Profile
Olivetol is incompatible with acid chlorides, acid anhydrides and oxidizing agents.
Fire Hazard
Olivetol is probably combustible.
Biological Activity
Olivetol (5-Pentylresorcinol, 5-n-Amylresorcinol) is a naturally occurring organic compound being a precursor in various syntheses of tetrahydrocannabinol. It acts as a competitive inhibitor of the cannabinoid receptors CB1 and CB2.
Safety
Olivetol is still a very new product. It has not been researched nearly enough to prove it's safe to use. So far, no one has reported any adverse side effects as far as we know. Until more information is available, carefully control your THC intake instead of relying on an untested gelcap.
Mode of action
When you look at olivetol's molecular structure, it'll look very familiar. It's as if someone took a THC molecule and sliced it in two. Olivetol, like THC, works by binding with the CB1 receptors that exist all over your body and brain. But, olivetol is thought to be smaller and stickier than THC, so that helps it reduce a raging high in two ways. First, it slips into any open receptors before THC can get there to block them—so you won't get any higher. Second, it bumps into THC molecules that are already lodged in a CB1 receptor to knock them loose and take their place. That's how it brings you down.
According to numerous personal accounts, olivetol works, but we don't know exactly how it does so. More research and investigation is needed, but in theory it's very similar to how Narcan acts to reverse opiate overdoses.
Properties of Olivetol
| Melting point: | 46-48 °C(lit.) |
| Boiling point: | 164 °C |
| Density | 1.068±0.06 g/cm3(Predicted) |
| Flash point: | >230 °F |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.59±0.10(Predicted) |
| color | Colourless to Beige |
| Stability: | Light Sensitive |
| InChI | InChI=1S/C11H16O2/c1-2-3-4-5-9-6-10(12)8-11(13)7-9/h6-8,12-13H,2-5H2,1H3 |
| CAS DataBase Reference | 500-66-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,3-Benzenediol, 5-pentyl-(500-66-3) |
| EPA Substance Registry System | Olivetol (500-66-3) |
Safety information for Olivetol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Olivetol
| InChIKey | IRMPFYJSHJGOPE-UHFFFAOYSA-N |
| SMILES | C1(O)=CC(CCCCC)=CC(O)=C1 |
Olivetol manufacturer
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole ELECTROLYTIC IRON POWDER 2-Methyl-2-phenylpropyl acetate 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate 4-Ethylbenzylamine Methyl 5-bromo-2-chloro-3-nitrobenzoate N-(5-Amino-2-methylphenyl)acetamide 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 5-Fluoro-2-Oxindole methyl L-alaninate hydrochloride diethyl L-glutamate hydrochloride Ethyl tosylcarbamateRelated products of tetrahydrofuran
You may like
-
 500-66-3 98%View Details
500-66-3 98%View Details
500-66-3 -
 Olivetol, 95% (Custom work) 500-66-3 99%View Details
Olivetol, 95% (Custom work) 500-66-3 99%View Details
500-66-3 -
 Olivetol 98% (GC) CAS 500-66-3View Details
Olivetol 98% (GC) CAS 500-66-3View Details
500-66-3 -
 Olivetol 99%View Details
Olivetol 99%View Details
500-66-3 -
 Olivetol 99%View Details
Olivetol 99%View Details -
 500-66-3 Olivetol 98%View Details
500-66-3 Olivetol 98%View Details
500-66-3 -
 Olivetol 98% CAS 500-66-3View Details
Olivetol 98% CAS 500-66-3View Details
500-66-3 -
 Olivetol CAS 500-66-3View Details
Olivetol CAS 500-66-3View Details
500-66-3