Glycerol
Synonym(s):Glycerin;Glycerol;1,2,3-Propanetriol;1,2,3-Propanetriol, Glycerine;1,2,3-Propanetriol, Trihydroxylpropane, Protol, Glycerin
- CAS NO.:56-81-5
- Empirical Formula: C3H8O3
- Molecular Weight: 92.09
- MDL number: MFCD00675440
- EINECS: 200-289-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
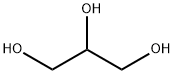
What is Glycerol?
Absorption
Well absorbed orally, poorly absorbed rectally. Studies in humans and animals indicate glycerol is rapidly absorbed in the intestine and the stomach
Toxicity
Glycerol has very low toxicity when ingested ; Rat LD50 (oral)-12600mg/kg Mice LD50 (oral )-4090mg/kg Human TDLo (oral) - 1428mg/kg
Description
Glycerol (also called glycerin or glycerine) is an alcohol produced by the hydrolysis of triglycerides, or as a byproduct during the manufacture of soap and biodiesel. It absorbs water from the air, and it is used as a moisturizer in soaps and lotions. Glycerol has a sweet taste, and it can be used as a food preservative and a nonsugar sweetener.
Chemical properties
Glycerin is the polyhydric alcohol 1,2,3 propanetriol [HOCH2-CH(OH)CH2OH] also known as glycerol. A clear, colorless, syrupy
liquid having a sweet taste. It has not more than a slight
characteristic odor, which is neither harsh nor disagreeable. It is
hygroscopic and its solutions are neutral. Glycerol is miscible with
water and with alcohol. It is insoluble in chloroform, in ether, and
in fixed and volatile oils.
Occurrence
Reported found in cocoa, apple, cider, beer, sour cherries, peach and wine
History
Glycerol was first isolated from olive oil and lead oxide by the Swedish chemist Carl Scheele (1742–1786) while making lead plaster soap in 1779. Scheele eventually realized that glycerol was a common ingredient in fats and oils and referred to glycerol as “the sweet principle of fats.” In 1811, the French chemist Michel Eugene Chevreul (1786–1889), who was a pioneer in the study of fats and oils, proposed the name glycerine after the Greek word glucos, which means sweet. Chevreul decomposed soaps isolating different acids such as stearic and butyric acid and discovered that glycerol was liberated when oils and fats were boiled in a basic mixture. Th éophile-Jules Pelouze (1807–1867) derived glycerol’s empirical formula in 1836.
The Uses of Glycerol
The three hydroxyl groups in glycerol allow extensive hydrogen bonding that gives glycerol its characteristic syrupy viscous texture and hygroscopic character. Approximately 40% of glycerol's use is for personal care products such as cosmetics, soaps, shampoos, lotions, mouthwash, and toothpaste. Glycerol's hygroscopic properties make it a good moisturizer in skin products.
In the food industry glycerol is used as a moistening agent, as a solvent for food coloring and syrups, to prevent crystallization of sugar in candies and icings, as a preservative, and as a sweetening agent. Approximately 10% of glycerol's use goes into tobacco processing, where it is sprayed on tobacco leaves before they are shredded to serve as a moistening agent.
The remaining 25% of glycerol's use is distributed among various industrial uses. It is used in cough syrups and elixir medicines. In industry, glycerol is found in lubricants, plasticizers, adhesives, antifreezes, resins, and insulating foams.
Background
A trihydroxy sugar alcohol that is an intermediate in carbohydrate and lipid metabolism.
Indications
It is used as a solvent, emollient, pharmaceutical agent, and sweetening agent.
Definition
ChEBI: A triol with a structure of propane substituted at positions 1, 2 and 3 by hydroxy groups.
Production Methods
Glycerin is mainly obtained from oils and fats as a by-product in the manufacture of soaps and fatty acids. It may also be obtained from natural sources by fermentation of, for example, sugar beet molasses in the presence of large quantities of sodium sulfite. Synthetically, glycerin may be prepared by the chlorination and saponification of propylene.
Preparation
Glycerol is the polyhydric alcohol most widely used for the
preparation of alkyd resins and is obtained both synthetically and as a byproduct
in the manufacture of soap. Most synthetic glycerol is obtained from
propylene via allyl chloride:
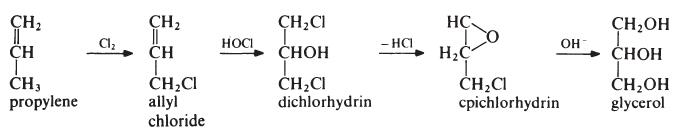
The first step is the 'hot' chlorination of propylene. A mixture of propylen(, and chlorine (4: 1 molar) is heated at about 500??C and 0.2 MPa (2 atmospheres). Under these conditions a free radical substitution reaction occurs rather than addition at the double bond and allyl chloride is the main product. This is treated with pre-formed hypochlorous acid (formed in a separate reactor by passing chlorine into water) at about 30??C to give the addition product, dichlorhydrin. The reaction mixture separates into two layers. The aqueous layer is removed to leave dichlorhydrin which is then stirred with a lime slurry to give epichlorhydrin. The epichlorhydrin is then hydrolysed to glycerol by treatment with aqueous sodium hydroxide at 150??C. The glycerol is produced as a dilute solution containing sodium chloride. Most of the water is evaporated off, during which operation the salt crystallizes out and is removed from the bottom of the evaporator to leave crude glycerol. The crude product is purified by distillation under reduced pressure. It may be noted that the intermediate epichlorhydrin has significance in its own right for the manufacture of epoxy resins.
Reactions
Glycerol reacts (1) with phosphorus pentachloride to form glyceryl trichloride, CH2Cl·CHCl · CH2Cl, (2) with acids to form esters, e.g., glycerol monoacetate CH2OH ·CHOH·CH2OOCCH3, glycerol diacetate C3H5(OH)(OCOCH3)2, glycerol triacetate (triacetin), CH2OOCCH3·CHOOCCH3·CH2OOCCH3, glycerol mononitrates (alpha, CH2OH·CHOH·CH2ONO2; beta, CH2OH · CHONO2·CH2OH), glycerol dinitrates (1, 2,CH2OH· CHONO2·CH2 ONO2; 1, 3,CH2ONO2·CHOH·CH2ONO2), glyceryl trinitrate (“nitroglycerine”), CH2ONO2·CHONO2·CH2ONO2, glyceryl tristearate (tristearin), CH2OOCC17H35·CHOO-CC17H35·CH2OOCC17H35, indirectly, glycerol monophosphates (alpha, CH2OH·CHOH·CH2OPO(OH)2, beta, CH2OH·CHOPO(OH)2·CH2OH, (3) with oxidizing agents, e.g., dilute nitric acid, to form glyceric acid, CH2OH·CHOH·COOH, tartaric acid, COOH·CHOH·COOH, mesoxalic acid, COOH·CO·COOH, (4) with phosphorus plus iodine, to form allyl iodide, CH2 : CHCH2I, which with hydrogen iodide yields propylene, CH2 : CHCH3, and then iso-propyl iodide, CH3CHICH3, (5) with sodium or sodium hydroxide to form alcoholates, (6) with sodium hydrogen sulfate or phosphorus pentoxide heated, to form acrolein, CH2 : CHCHO. Glycide alcohol is obtained by treatment of glycerol alphamonochlorohydrin CH2OH·CHOH·CH2Cl, which is made by reaction of hypochlorous acid and allyl alcohol with barium hydroxide. With hydrogen chloride, glycide alcohol yields epichlorohydrin.
Aroma threshold values
Greater than 20,000 ppm.
Synthesis Reference(s)
Tetrahedron Letters, 32, p. 187, 1991 DOI: 10.1016/0040-4039(91)80850-6
General Description
A colorless to brown colored liquid. Combustible but may require some effort to ignite. Residual sodium hydroxide (lye) causes crude material to be corrosive to metals and/or tissue.
Air & Water Reactions
Hygroscopic. Water soluble.
Reactivity Profile
GLYCERINE is incompatible with strong oxidizers. Glycerol is also incompatible with hydrogen peroxide, potassium permanganate, nitric acid + sulfuric acid, perchloric acid + lead oxide, acetic anhydride, aniline + nitrobenzene, Ca(OCl)2, CrO3, F2 + PbO, KMnO4, K2O2, AgClO4 and NaH. A mixture with chlorine explodes if heated to 158-176° F. Glycerol reacts with acetic acid, potassium peroxide, sodium peroxide, hydrochloric acid, (HClO4 + PbO) and Na2O2. Contact with potassium chlorate may be explosive. Glycerol also reacts with ethylene oxide, perchloric acid, nitric acid + hydrofluoric acid and phosphorus triiodide.
Health Hazard
No hazard
Fire Hazard
Glycerol is combustible.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Pharmaceutical Applications
Glycerin is used in a wide variety of pharmaceutical formulations
including oral, otic, ophthalmic, topical, and parenteral preparations.
In topical pharmaceutical formulations and cosmetics, glycerin is
used primarily for its humectant and emollient properties. Glycerin
is used as a solvent or cosolvent in creams and emulsions.
Glycerin is additionally used in aqueous and nonaqueous gels and
also as an additive in patch applications. In parenteral
formulations, glycerin is used mainly as a solvent and cosolvent.
In oral solutions, glycerin is used as a solvent, sweetening
agent, antimicrobial preservative, and viscosity-increasing agent. It
is also used as a plasticizer and in film coatings.
Glycerin is used as a plasticizer of gelatin in the production of
soft-gelatin capsules and gelatin suppositories.
Glycerin is employed as a therapeutic agent in a variety of
clinical applications, and is also used as a food additive.
Biochem/physiol Actions
Glycerol is hygroscopic in nature and is soluble in water owing to its three hydrophilic alcoholic hydroxyl groups. It can form both inter- and intramolecular hydrogen bonds, making it a very flexible molecule. The physiologic effect of glycerine is due to cell-mediated immunity, increased IgG production and increased histamine release.
Pharmacokinetics
Glycerin is commonly classified as an osmotic laxative but may act additionally or alternatively through its local irritant effects; it may also have lubricating and fecal softening actions. Glycerin suppositories usually work within 15 to 30 minutes.
Safety Profile
Poison by subcutaneous route. Mildly toxic by ingestion. Human systemic effects by ingestion: headache and nausea or vomiting. Experimental reproductive effects. Human mutation data reported. A skin and eye irritant. In the form of mist it is a nuisance particulate and inhalation irritant. Combustible liquid when exposed to heat, flame, or powerful oxidizers. Mixtures with hydrogen peroxide are highly explosive. Ignites on contact with potassium permanganate, calcium hypochlorite. Mixture with nitric acid + sulfuric acid forms the explosive glyceql nitrate. Mixture with perchloric acid + lead oxide forms explosive perchlorate esters. Confined mixture with chlorine explodes if heated to 70-80'. Can react violently with acetic anhydride, aniline + nitrobenzene, Ca(OCl)2, Cr03,Cr203, F2 + PbO, phosphorus triiodide, ethylene oxide + heat, KMnO4, K2O2, AgClO4, Na2O2, NaH. Energetic reaction with sodium hydride. Mixture with nitric acid + hydrofluoric acid is a storage hazard due to gas evolution. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes.
Safety
When used as an excipient or food additive, glycerin is not
usually associated with any adverse effects and is generally regarded
as a nontoxic and nonirritant material.
LD50 (guinea pig, oral): 7.75 g/kg
LD50 (mouse, IP): 8.70 g/kg
LD50 (mouse, IV): 4.25 g/kg
LD50 (mouse, oral): 4.1 g/kg
LD50 (mouse, SC): 0.09 g/kg
LD50 (rabbit, IV): 0.05 g/kg
LD50 (rabbit, oral): 27 g/kg
LD50 (rat, IP): 4.42 g/kg
LD50 (rat, oral): 5.57 g/kg
LD50 (rat, oral): 12.6 g/kg
LD50 (rat, SC): 0.1 g/kg
Synthesis
Obtained from oils and fats as a by-product in the manufacture of soaps and fatty acids; synthesized from propylene; also production from sugars by fermentation.
Potential Exposure
Glycerol is used as a humectant in tobacco; it is used in cosmetics, antifreezes and inks. It is used as a fiber lubricant. It is used as a raw material for alkyd resins and in explosives manufacture.
Environmental Fate
Glycerol is completely miscible with water. When exposed to moist air, it absorbs water (hydroscopic) as well as gasses such as hydrogen sulfide and sulfur dioxide. Glycerol has low volatility, with a vapor pressure of 0.000106 hPa at 25 ℃; the calculated Henry’s law constant (maximum solubility) is 9.75E-6 Pam3 mol-1. The calculated photodegradation halflife of glycerol in air is 6.8 h. Glycerol is readily biodegradable. When released to the environment, glycerol is distributed to water, with negligible amounts distributed in air, soil, or sediment. Based on a log Kow of -1.76, glycerol has a low bioaccumulation potential and is not expected to bioaccumulate.
Metabolism
Glycerin is a substrate for synthesis of triacylglycerols and of phospholipids in the liver and adipose tissue. When fat metabolized as a source of energy, glycerol and fatty acids are released into the bloodstream. Circulating glycerin does not glycate proteins and does not lead to the formation of advanced glycation endproducts (AGEs). In some organisms, the glycerin component can enter the glycolysis pathway directly to provide a substrate for energy or glucose production. Glycerol must be converted to their intermediate glyceraldehyde 3-phosphate before being used in glycolysis or gluconeogenesis. Glycerol metabolism is regulated by the enzymes glycerol kinase, (cytosolic) NAD+-dependent G3P dehydrogenase and (mitochondrial) FAD-linked G3P dehydrogenase.
Storage
Glycerin is hygroscopic. Pure glycerin is not prone to oxidation by the atmosphere under ordinary storage conditions, but it decomposes on heating with the evolution of toxic acrolein. Mixtures of glycerin with water, ethanol (95%), and propylene glycol are chemically stable.
Effectiveness
Glycerol is effective for:
Constipation. Using glycerol as a suppository or as an enema in the rectum decreases constipation in adults and children at least 2 years of age.
An inherited skin disorder that causes dry, scaly skin (ichthyosis). Applying a specific product containing glycerol and paraffin to the skin reduces symptoms like itching and scales in children with ichthyosis.
Shipping
UN1760 Corrosive liquids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, Technical Name Required.
Toxicity evaluation
The medicinal actions of glycerol are due to osmotic action; orally, 75 ml of glycerol is equivalent to 996 mosmol. Large intravenous doses of glycerol can cause hemolysis, hemoglobinuria, and subsequent renal failure. The osmotic effect of glycerol can lead to tissue dehydration and decreased cerebrospinal fluid pressure. Certain medical conditions such as cardiac, renal, or liver disease and/or diabetes mellitus may be exacerbated by shifts in body water as a result of oral, intravenous, or rectal (suppositories) administration of glycerol. No systemic effects have been reported following the application of large amounts of glycerol to the skin.
Incompatibilities
Glycerin may explode if mixed with strong oxidizing agents such as
chromium trioxide, potassium chlorate, or potassium permanganate.
In dilute solution, the reaction proceeds at a slower rate with
several oxidation products being formed. Black discoloration of
glycerin occurs in the presence of light, or on contact with zinc oxide
or basic bismuth nitrate.
An iron contaminant in glycerin is responsible for the darkening
in color of mixtures containing phenols, salicylates, and tannin.
Glycerin forms a boric acid complex, glyceroboric acid, that is a
stronger acid than boric acid.
Waste Disposal
Mixture with a more flamma ble solvent followed by incineration.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (dental pastes; buccal preparations; inhalations; injections; nasal and ophthalmic preparations; oral capsules, solutions, suspensions and tablets; otic, rectal, topical, transdermal, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Glycerol
| Melting point: | 20 °C(lit.) |
| Boiling point: | 290 °C |
| Density | 1.25 g/mL(lit.) |
| vapor density | 3.1 (vs air) |
| vapor pressure | <1 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 2525 | GLYCEROL |
| Flash point: | 320 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 5 M at 20 °C, clear, colorless |
| form | Viscous Liquid |
| pka | 14.15(at 25℃) |
| Specific Gravity | 1.265 (15/15℃)1.262 |
| color | APHA: ≤10 |
| Odor | Odorless. |
| PH Range | 5.5 - 8 |
| PH | 5.5-8 (25℃, 5M in H2O) |
| explosive limit | 2.6-11.3%(V) |
| Water Solubility | >500 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.04 |
| JECFA Number | 909 |
| Merck | 14,4484 |
| BRN | 635685 |
| Dielectric constant | 47.0(Ambient) |
| Exposure limits | OSHA: TWA 15 mg/m3; TWA 5 mg/m3 |
| Stability: | Stable. Incompatible with perchloric acid, lead oxide, acetic anhydride, nitrobenzene, chlorine, peroxides, strong acids, strong bases. Combustible. |
| CAS DataBase Reference | 56-81-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2,3-Propanetriol(56-81-5) |
| EPA Substance Registry System | Glycerine (56-81-5) |
Safety information for Glycerol
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H320:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for Glycerol
| InChIKey | PEDCQBHIVMGVHV-UHFFFAOYSA-N |
Glycerol manufacturer
Shiv Shakti Trading Corporation
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Glycerine 98%View Details
Glycerine 98%View Details -
 Glycerine 99%View Details
Glycerine 99%View Details -
 Glycerine 98%View Details
Glycerine 98%View Details -
 Glycerine 98%View Details
Glycerine 98%View Details -
 Glycerine 99%View Details
Glycerine 99%View Details -
 GLYCERINE 98%View Details
GLYCERINE 98%View Details -
 Glycerine 99%View Details
Glycerine 99%View Details -
 Glycerine CASView Details
Glycerine CASView Details
