Octyltrichlorosilane
Synonym(s):Octyltrichlorosilane
- CAS NO.:5283-66-9
- Empirical Formula: C8H17Cl3Si
- Molecular Weight: 247.66
- MDL number: MFCD00000488
- EINECS: 226-112-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-19 17:50:00
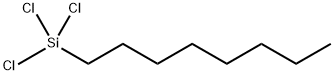
What is Octyltrichlorosilane?
Chemical properties
CLEAR COLOURLESS TO LIGHT YELLOW LIQUID
The Uses of Octyltrichlorosilane
OTS functionalized magnesium oxide (MnO2)/aluminium oxide (Al2O3) surface can be used as a catalyst in the decomposition of ozone (O3) and nitrogen dioxide (NO2). OTS can also be used for the surface modification of silicon oxide (SiO2), used in the fabrication of pentacene organic field effect transistors (OFETs). OTS functionalized wafers can be coated on enhanced green fluorescent protein (GFP) and anchor peptides based films to determine the thickness of the films.
General Description
N-Octyltrichlorosilane is a colorless liquid with a pungent odor. It is decomposed by water to hydrochloric acid with evolution of heat. Octyltrichlorosilane is corrosive to metals and tissue and used as an intermediate for silicones.
Reactivity Profile
Chlorosilanes, such as N-OCTYLTRICHLOROSILANE, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Flammability and Explosibility
Not classified
Safety Profile
A corrosive irritant to skin, eyes, and mucous membranes. Will react with water or steam to produce toxic and corrosive fumes. When heated to decomposition it emits toxic fumes of Cl-. See also CHLOROSILANES.
Synthesis
In a 25 ml oven dried stainless steel tube, 0.22 g (0.67 mmol) of benzyltributylphosphonium chloride, 1.00 g (6.73 mmol) of 1-chlorooctane, and 2.71 g (20.0 mmol) of trichlorosilane were added under a dry nitrogen atmosphere. After sealing the cylinder with a cap, the reactor was maintained at 170°C. for 2 hrs. The resulting mixture was distilled to yield 1.45 g of n-octyltrichlorosilane (yield; 87%). n-Octyltrichlorosilane, 1.45 g, 87% yield MS (70eV EI) m/z (relative intensity): 250(1), 248(3), 246(4), 179(12), 177(35), 175 (34), 135(53), 133(54), 85(100), 71(57), 57(98).
Purification Methods
Purify the silane by repeated fractionation using a 15-20 theoretical plate glass column packed with glass helices. This can be done more efficiently using a spinning band column. The purity can be checked by analysing for Cl (ca 0.5-1g of sample is dissolved in 25mL of MeOH, diluted with H2O and titrated with standard alkali). It is moisture sensitive. [Whitmore J Am Chem Soc 68 475 1946, El-Abbady & Anderson J Am Chem Soc 80 1737 1958, Beilstein 4 III 1907.]
Properties of Octyltrichlorosilane
| Boiling point: | 233 °C731 mm Hg(lit.) |
| Density | 1.07 g/mL at 25 °C(lit.) |
| vapor density | >1 (vs air) |
| vapor pressure | 0-3.5Pa at 25℃ |
| refractive index | n |
| Flash point: | 206 °F |
| form | clear liquid |
| Specific Gravity | 1.074 |
| color | Fuming liquid |
| Water Solubility | 59g/L at 20℃ |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 1744230 |
| CAS DataBase Reference | 5283-66-9(CAS DataBase Reference) |
| EPA Substance Registry System | Silane, trichlorooctyl- (5283-66-9) |
Safety information for Octyltrichlorosilane
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Octyltrichlorosilane
| InChIKey | RCHUVCPBWWSUMC-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
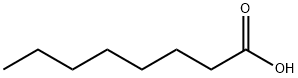
 n-Octyltrichlorosilane CAS 5283-66-9View Details
n-Octyltrichlorosilane CAS 5283-66-9View Details
5283-66-9 -
 n-Octyltrichlorosilane CAS 5283-66-9View Details
n-Octyltrichlorosilane CAS 5283-66-9View Details
5283-66-9 -
 TRICHLOROOCTYL SILANE CAS 5283-66-9View Details
TRICHLOROOCTYL SILANE CAS 5283-66-9View Details
5283-66-9 -
 Trichloro(octyl)silane CAS 5283-66-9View Details
Trichloro(octyl)silane CAS 5283-66-9View Details
5283-66-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1