O-Phospho-L-serine
Synonym(s):L -2-Amino-3-hydroxypropanoic acid 3-phosphate;L -Serine O-phosphate;L -SOP;L-2-Amino-3-hydroxypropanoic acid 3-phosphate, L-SOP, L-Serine O-phosphate;Monoclonal Anti-Phosphoserine
- CAS NO.:407-41-0
- Empirical Formula: C3H8NO6P
- Molecular Weight: 185.07
- MDL number: MFCD00065935
- EINECS: 206-986-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
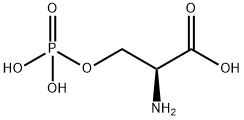
What is O-Phospho-L-serine?
Description
O-
Description
L-O-Phosphoserine is a biomolecule that is used in combination with?L-glutamine and vitamin B12?as a “roborant”—a restorative tonic—marketed under the trade names Vitasprint B12?and Fosforina B12. It is biosynthesized via the enzymatic phosphorylation of?L-serine. F. C. Neuhaus and S. Korkes described the preparation of the racemic mixture in 1958. In 2011, C. J. Noren, J. Rinehart, and D. S?ll developed?a way to genetically encode L-O-phosphoserine?anywhere in a protein sequence.
Chemical properties
White powder
The Uses of O-Phospho-L-serine
O-Phospho-L-Serine is an agonist of the group III metabotropic glutamate receptors mGluR4a and mGluR6 (EC50s = 2-5 μM). It mimics the phosphatidylserine head group and has been shown to inhibit the proliferation of microglia and to enhance neuronal differentiation of progenitor cells.
The Uses of O-Phospho-L-serine
roborant
The Uses of O-Phospho-L-serine
L-O-Phosphoserine is the catalytic domain of human protein kinase C β II when complexed with a malemide inhibitor. It is also used in the purification of the Epstein-Barr virus nuclear antigen 2A.
What are the applications of Application
O-Phospho-L-serine is a competitive NMDA antagonist/ group III mGluR agonist
Definition
ChEBI: O-phospho-L-serine is the L-enantiomer of O-phosphoserine. It has a role as an EC 1.4.7.1 [glutamate synthase (ferredoxin)] inhibitor, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, an EC 2.5.1.49 (O-acetylhomoserine aminocarboxypropyltransferase) inhibitor, an EC 4.3.1.10 (serine-sulfate ammonia-lyase) inhibitor and a mouse metabolite. It is a conjugate acid of an O-phosphonato-L-serine(2-). It is an enantiomer of an O-phospho-D-serine.
General Description
Monoclonal Anti-Phosphoserine (mouse IgG1 isotype) is derived from the hybridoma produced by the fusion of mouse myeloma cells and splenocytes from an immunized mouse.
Flammability and Explosibility
Not classified
Biological Activity
Group III metabotropic glutamate receptor agonist; analog of L-AP4 (L-(+)-2-Amino-4-phosphonobutyric acid ). Inhibits proliferation and enhances neuronal differentiation in progenitor cells.
Biochem/physiol Actions
Protein phosphorylation and dephosphorylation are basic signaling mechanisms that modify protein function in eukaryotic cells. Phosphorylation is a rare posttranslational event in normal tissues, however, the abundance of phosphorylated cellular proteins increases several folds following various activation processes. The main amino acids that are phosphorylated are tyrosine, serine, or threonine (pTyr/pSer/pThr), each having specific kinases that phosphorylate them and specific phosphatases that dephosphorylate them. Mono and polyclonal antibodies directed against phosphorylated residues were generated and found useful as analytical and preparative tools by enabling the identification, quantification, and immunoaffinity isolation of phosphorylated cellular proteins.
storage
Room temperature
Purification Methods
Recrystallise the phospho-serine by dissolving 10g in H2O (150mL) at 25o, stirring for up to 20minutes. Undissolved material is filtered off (Büchner), and 95% EtOH (85mL) is added dropwise during 4minutes, and set aside at 25o for 3hours, then at 3o overnight. The crystals are washed with 95% EtOH (100mL), then dry Et2O (50mL), and dried in a vacuum (yield 6.5g). A further quantity (1.5g) can be obtained by keeping the mother liquors and washings at -10o for 1 week. The DL-isomer has m 167-170o(dec) after recrystallisation from H2O/EtOH or MeOH. [Neuhaus & Korkes Biochemical Preparations 6 75 1958, Neuhaus & Byrne J Biol Chem 234 113 1959, IR: F.lsch & Mellander Acta Chem Scand 11 1232 1957, Beilstein 4 IV 3120.]
Properties of O-Phospho-L-serine
| Melting point: | 190 °C(lit.) |
| Boiling point: | 475.4±55.0 °C(Predicted) |
| alpha | [α]D20 +14~+18°(c=5,dil.HCl) |
| Density | 1.809±0.06 g/cm3(Predicted) |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | under inert gas |
| solubility | H2O: 50 mg/mL hot, clear, colorless to slightly yellow |
| pka | pK1:2.08;pK2:5.65;pK3:9.74 (25°C) |
| form | Crystalline Powder |
| color | White |
| Water Solubility | 28.34g/L at 20℃ |
| Merck | 14,7363 |
| BRN | 1726826 |
| CAS DataBase Reference | 407-41-0(CAS DataBase Reference) |
Safety information for O-Phospho-L-serine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for O-Phospho-L-serine
| InChIKey | BZQFBWGGLXLEPQ-UWTATZPHSA-N |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 O-Phospho-l-serine 95% CAS 407-41-0View Details
O-Phospho-l-serine 95% CAS 407-41-0View Details
407-41-0 -
 L-O-Phosphoserine CAS 407-41-0View Details
L-O-Phosphoserine CAS 407-41-0View Details
407-41-0 -
 Monoclonal Anti-Phosphoserine antibody produced in mouse CASView Details
Monoclonal Anti-Phosphoserine antibody produced in mouse CASView Details -
 Monoclonal Anti-Phosphoserine antibody produced in mouse CASView Details
Monoclonal Anti-Phosphoserine antibody produced in mouse CASView Details -
 O-Phospho-L-serine CAS 407-41-0View Details
O-Phospho-L-serine CAS 407-41-0View Details
407-41-0 -
 Monoclonal Anti-Phosphoserine−Agarose antibody produced in mouse CASView Details
Monoclonal Anti-Phosphoserine−Agarose antibody produced in mouse CASView Details -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1