L-Homoserine
Synonym(s):(S)-2-Amino-4-hydroxybutyric acid;Hse;L-(-)-Homoserine
- CAS NO.:672-15-1
- Empirical Formula: C4H9NO3
- Molecular Weight: 119.12
- MDL number: MFCD00063090
- EINECS: 211-590-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
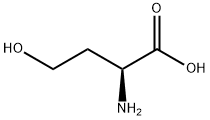
What is L-Homoserine?
Chemical properties
white to light yellow crystalline powder
The Uses of L-Homoserine
L-Homoserine is used in the biosynthesis of methionine, threonine and isoleucine. It is also used as an internal standard for neurotransmitter analysis and amino acids quantification.
Definition
ChEBI: L-homoserine is the L-enantiomer of homoserine. It has a role as a human metabolite, an algal metabolite, a Saccharomyces cerevisiae metabolite and an Escherichia coli metabolite. It is an enantiomer of a D-homoserine. It is a tautomer of a L-homoserine zwitterion.
Synthesis Reference(s)
Journal of the American Chemical Society, 80, p. 3147, 1958 DOI: 10.1021/ja01545a057
The Journal of Organic Chemistry, 24, p. 1794, 1959 DOI: 10.1021/jo01093a610
General Description
L-Homoserine is a variant of serine with an additional carbon on its side chain.
Biochem/physiol Actions
L-Homoserine is synthesized by deoxidation process, catalysed by homoserine dehydrogenase. This is one of the steps in the synthesis of L-threonine. The carbon flux in in bacteria such as E. coli is maintained by this reaction.
Purification Methods
Likely impurities are N-chloroacetyl-L-homoserine, N-chloroacetyl-D-homoserine, L-homoserine, homoserine lactone, homoserine anhydride (formed in strong solutions of homoserine if slightly acidic). It cyclises to the lactone in strongly acidic solution. It crystallises from water by adding 9 volumes of EtOH. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2612-2616 1961, Beilstein 4 IV 3187.]
Properties of L-Homoserine
| Melting point: | 203 °C (dec.)(lit.) |
| Boiling point: | 222.38°C (rough estimate) |
| alpha | -8.5 º (c=2, H2O 22 ºC) |
| Density | 1.3126 (rough estimate) |
| refractive index | 1.4183 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | 1100g/l |
| form | Crystalline Powder |
| pka | 2.71(at 25℃) |
| color | White to light yellow |
| Water Solubility | 1100 g/L (30 ºC) |
| Merck | 14,4739 |
| BRN | 1721681 |
| CAS DataBase Reference | 672-15-1(CAS DataBase Reference) |
Safety information for L-Homoserine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for L-Homoserine
| InChIKey | UKAUYVFTDYCKQA-VKHMYHEASA-N |
L-Homoserine manufacturer
JSK Chemicals
Sami Sabinsa Group Limited (formerly Sami Labs Limited)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


![2-[(2-CHLOROACETYL)AMINO]SUCCINIC ACID](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB7133048.gif)
![DIMETHYL 5-(2-([3-CHLORO-5-(TRIFLUOROMETHYL)-2-PYRIDINYL]AMINO)ETHYL)-4,6-DIOXOTETRAHYDRO-2H-PYRROLO[3,4-D]ISOXAZOLE-3,3(3AH)-DICARBOXYLATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB9738036.gif)
![DIMETHYL 5-(2,4-DICHLOROPHENYL)-4,6-DIOXOTETRAHYDRO-2H-PYRROLO[3,4-D]ISOXAZOLE-3,3(3AH)-DICARBOXYLATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure3/GIF/CB1753819.gif)
You may like
-
 L-Homoserine CAS 672-15-1View Details
L-Homoserine CAS 672-15-1View Details
672-15-1 -
 L-Homoserine CAS 672-15-1View Details
L-Homoserine CAS 672-15-1View Details
672-15-1 -
 L-Homoserine 98% CAS 672-15-1View Details
L-Homoserine 98% CAS 672-15-1View Details
672-15-1 -
 L-(-)-Homoserine CAS 672-15-1View Details
L-(-)-Homoserine CAS 672-15-1View Details
672-15-1 -
 L-Homoserine CAS 672-15-1View Details
L-Homoserine CAS 672-15-1View Details
672-15-1 -
 672-15-1 L-Homoserine,98% 99%View Details
672-15-1 L-Homoserine,98% 99%View Details
672-15-1 -
 672-15-1 98%View Details
672-15-1 98%View Details
672-15-1 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1