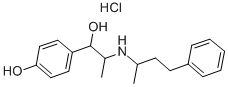
Nylidrin
- CAS NO.:447-41-6
- Empirical Formula: C19H25NO2
- Molecular Weight: 299.4073
- MDL number: MFCD00599550
- EINECS: 207-182-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-21 10:59:17
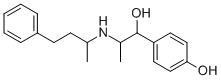
What is Nylidrin?
Absorption
Readily absorbed from the gastrointestinal tract .
Toxicity
Adverse effects of oral sympathomimetics, such as Nylidrin, are common. The most frequent effects are hypertension, palpitations, nausea, anxiety, mydriasis, and restlessness/agitation .
Large overdoses and severe toxicity may lead to seizures, hallucinations, agitated delirium, and tachydysrhythmias including
supraventricular tachycardia and ventricular tachycardia. Vasospasm can lead to myocardial ischemia focal cerebrovascular deficits. Severe hypertension may also result in intracranial hemorrhage or renal insufficiency. Reflex bradycardia because of significant hypertension is possible. Prolonged agitation can lead to rhabdomyolysis and hyperthermia .
Originator
Arlidin,U.S.V. ,US,1955
The Uses of Nylidrin
Buphenin (Nylidrin), is shown to be an effective inhibitor of IgE-mediated release of histamine from human basophils, and thus can be used as antiallergic agent. It is also one of the FDA approved drug as Inhibitors of the Human Sodium Taurocholate Cotransporting Polypeptide (NTCP).
Indications
Nylidrin is mainly indicated in conditions like arteriosclerosis, cerebrovascular disease, peripheral vascular disease, Raynaud's disease, thrombo-angitis obliterans, and thrombophlebitis . It may sometimes be used in the treatment of peripheral vascular disorders in addition premature labor (however, the drug is not approved for premature labor).
Background
Nylidrin, also known as buphenine belongs to the category of drugs called vasodilators, which relax blood vessels and increase blood flow. Nylidrin is a peripheral vasodilator. Some studies show the evidence of improving cognitive impairment in selected individuals, such as geriatric patients with mild to moderate symptoms of cognitive, emotional and physical impairment .
Nylidrin is utilized to treat several disorders that may benefit from increased blood flow (for example, certain mental disorders, blood vessel disease due to diabetes, frostbite, night leg cramps, and certain types of ulcers). This medication works by dilating (widening) blood vessels to help increase blood flow (improving circulation) throughout the body, including the extremities and central nervous system. This effect may help to improve memory/judgment, improve walking ability, and support the healing of frostbite/ulcers .
FDA has considered nylidrin as "lacking substantial evidence of effectiveness" in cerebral ischemia, cerebral arteriosclerosis, and other cerebral circulatory insufficiencies. Therefore, the FDA has withdrawn nylidrin from the U.S. market .
Definition
ChEBI: 4-[1-hydroxy-2-(4-phenylbutan-2-ylamino)propyl]phenol is an alkylbenzene.
Manufacturing Process
8 grams of the hydrobromide of 1-(p-benzoxyphenyl)-2-(α-methyl-γ-phenylpropylamino)-propanone-(1) were obtained by heating equivalent quantities of p-benzoxy-α-bromopropiophenone and 1-phenyl-3-amino-butane for an hour on the water bath in the absence of solvents. The product was purified by twice boiling with five times the quantity of acetic acid and filtration at 80°C, then shaken in contact with hydrogen with 0.8 gram of Raney nickel in 70 cc of pure methanol containing 0.96 gram (corresponding to 1 mol) of KOH. After 4 hours 2 mols of hydrogen had been taken up and the solution was filtered from the catalyst, evaporated in vacuo, and the residue triturated first with water to remove potassium bromide and then with methanol to remove potassium bromide. 3.7 grams (72% of the theoretical yield) of the compound specified, melting at 110° to 112°C, were obtained, as described in US Patent 2,661,373.
Therapeutic Function
Vasodilator
Pharmacokinetics
Nylidrin hydrochloride acts mainly by beta-receptor stimulation , . Beta stimulation with nylidrin has been studied and confirmed in a variety of isolated tissues from rabbits, guinea pigs, as well as dogs. This drug has been shown to dilate arterioles in skeletal muscle and to increase cardiac output in the anesthetized dog and cat as well as the unanesthetized man. An increase in cerebral blood flow and a decrease in vascular resistance has also been reported. The result of this combination of mechanisms is an improved blood supply to ischemic tissues, with minimal change in blood pressure (generally) .
Nylidrin causes peripheral vasodilation, a positive inotropic effect, and an increased volume of gastric acid. , . According to one study, there are two primary effects of this agent following the intra‐arterial route of administration: one, to decrease total peripheral resistance; and two, a direct effect on the heart tissue to increase cardiac output . It also acts directly on the arteries and arterioles of the skeletal muscles. Additionally, it suppresses uterine contractility thereby preventing or halting premature labor .
Metabolism
Not Available
Properties of Nylidrin
| Melting point: | 111-112° |
| Boiling point: | 476.0±40.0 °C(Predicted) |
| Density | 1.101±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMF: 30 mg/ml,DMSO: 30 mg/ml,DMSO:PBS (pH 7.2) (1:7): 0.12 mg/ml |
| form | A crystalline solid |
| pka | 9.96±0.26(Predicted) |
Safety information for Nylidrin
Computed Descriptors for Nylidrin
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran



You may like
-
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
 Cyclopentane carboxxylic acid 3400-45-1 99%View Details
Cyclopentane carboxxylic acid 3400-45-1 99%View Details
3400-45-1 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0