NUARIMOL
- CAS NO.:63284-71-9
- Empirical Formula: C17H12ClFN2O
- Molecular Weight: 314.74
- MDL number: MFCD00078677
- EINECS: 264-071-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:27:31
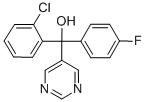
What is NUARIMOL?
The Uses of NUARIMOL
Nuarimol is a systemic fungicide with both curative and protective activity. It controls a wide range of pathogenic fungi, e.g. Cercosporella spp., Septoria spp., Ustilago spp., powdery mildews, leaf spot, etc. in cereals (as a foliar spray and as a seed treatment), powdery mildews on pome fruits, stone fruits, vines, cucurbits and other crops and scab on apples.
The Uses of NUARIMOL
Nuarimol is used in the preparation of difluoroethyl-containing heterocyclic compounds useful as antiviral agents and fungicides. Also used as a substance in the modelling inhibition of avian aromatase by azole pesticides.
Metabolic pathway
Photolytic degradation is the primary dissipation mechanism of nuarimol in the environment. Major degradation reactions observed on plants/soil surfaces and water include hydroxylation of the phenyl groups, oxidation of the carbinol carbon atom, dehalogenation and carbinol dehydroxylation (Scheme 1). The primary metabolic pathway of nuarimol in rats involves mainly aryl hydroxylation (Scheme 2).
Degradation
Nuarimol is stable to hydrolytic degradation when maintained in sterile
buffered solutions (pH 3,6 and 9) in the dark at 52 °C (Saunders, 1977). It
was readily degraded in distilled water via photolysis. The photolytic
DT50 of nuarimol was approximately 1 hour. Numerous photoproducts
were observed; however, no structural characterisation information was
reported (Zornes and Donoho, 1978).
Nuarimol was extensively photodegraded on solid surfaces. More than
80 photodegradation products were observed when nuarimol was
exposed to sunlight on a stainless steel surface for up to 150 hours (Althaus, 1980a). All photoproducts were formed at very low levels (less
than 3% each). The structures of 23 photoproducts were identified. An
abbreviated photodegradation pathway of nuarimol (based on products
accounting for 1% or greater) is presented in Scheme 1. These products
were generated from the following reactions: aryl hydroxylation of the
chlorophenyl and fluorophenyl moieties (to yield 2, 3), cleavage of the
pyrimidine ring and the oxidation of the carbinol carbon atom (4,5) and
dehydroxylation of the carbinol moiety (6). Carboxylic acid fragments
from the phenyl(7,8,9) and pyrimidine moieties (10), resulting from the
cleavage of the parent phenyl and pyrimidine linkages, were also
observed.
Properties of NUARIMOL
| Melting point: | 126°C |
| Boiling point: | 475.2±40.0 °C(Predicted) |
| Density | 1.3255 (estimate) |
| vapor pressure | 1 x 10-5 Pa at 23 °C |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | neat |
| Water Solubility | 26 mg l-1 (pH 7) at 25 °C |
| pka | 11.37±0.29(Predicted) |
| color | White to Pale Yellow |
| BRN | 6223667 |
| EPA Substance Registry System | Nuarimol (63284-71-9) |
Safety information for NUARIMOL
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for NUARIMOL
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Nuarimol CAS 63284-71-9View Details
Nuarimol CAS 63284-71-9View Details
63284-71-9 -
 Nuarimol CAS 63284-71-9View Details
Nuarimol CAS 63284-71-9View Details
63284-71-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
