Benzophenone
Synonym(s):Benzophenone;Diphenyl ketone;Diphenylketon, Diphenylmethanon, Benzoylbenzol;Diphenylmethanone;NSC 8077
- CAS NO.:119-61-9
- Empirical Formula: C13H10O
- Molecular Weight: 182.22
- MDL number: MFCD00003076
- EINECS: 204-337-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:53
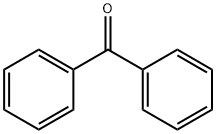
What is Benzophenone?
Description
Benzophenone is a combustible, white,crystalline solid with a rose-like odor. Molecularweight=182.23; Specific gravity (H2O:1) = 1.085 at 50℃;Boiling point = 305℃; Freezing/Melting point = 48.5℃;Latent heat of vaporization=2.93 3 105 J/kg; Heat ofcombustion= -358 3 105 J/kg. Hazard Identification(based on NFPA-704 M Rating System): Health 1,Flammability 1, Reactivity 0. Insoluble in water.
Chemical properties
Benzophenone is a combustible, white, crystalline solid with a rose-like odor. soluble in ethanol, ether, chloroform and other organic solvents and monomers, insoluble in water. It is a free radical photoinitiator, mainly used in free radical UV curing systems, such as coatings, inks, adhesives, etc. It can be prepared in several ways, for example, by Friedel–Crafts reaction of benzene and benzoyl chloride with aluminum chloride, or of benzene and carbon tetrachloride, and oxidation of diphenylmethane.
Occurrence
Benzophenone has been found in various fruits, including Vitis vinifera L., black tea, cherimoya (Annona cherimola), mountain papaya (Carica pubescens), and soursop (Annona muricata L.).
The Uses of Benzophenone
Benzophenone is used as a synthetic intermediate for manufacture of pharmaceuticals and agricultural chemicals. It is also used as a photoinitiator in UV-curable printing inks, as a fragrance in perfumes, as a flavor enhancer in foods. Benzophenone can be added as a UV-absorbing agent to plastics, lacquers, and coatings at concentrations of 2–8%.
Production Methods
Benzophenone is commercially synthesized by the atmospheric oxidation of diphenylmethane using a catalyst of copper naphthenate. Alternatively, it can be produced by a Friedel–Crafts acylation of benzene using either benzoyl chloride or phosgene in the presence of aluminum chloride .
Definition
ChEBI: Benzophenone is the simplest member of the class of benzophenones, being formaldehyde in which both hydrogens are replaced by phenyl groups. It has a role as a photosensitizing agent and a plant metabolite.
Synthesis Reference(s)
Tetrahedron Letters, 36, p. 2285, 1995 DOI: 10.1016/0040-4039(95)00191-E
Chemical and Pharmaceutical Bulletin, 34, p. 3595, 1986 DOI: 10.1248/cpb.34.3595
General Description
Benzophenone(119-61-9) appears as white solid with a flowery odor. May float or sink in water. It is a widely used building block in organic chemistry, being the parent diarylketone.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Ketones, such as Benzophenone, are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4. Benzophenone can react with oxidizing materials.
Health Hazard
Ingestion causes gastrointestinal disturbances. Contact causes eye irritation and, if prolonged, irritation of skin.
Fire Hazard
Flash point data for Benzophenone are not available, but Benzophenone is probably combustible.
Contact allergens
Unsubstituted benzophenone is largely used in chemical applications. It acts as a marker for photoallergy to ketoprofen.
Safety Profile
Moderately toxic by ingestion andintraperitoneal routes. Combustible when heated.Incompatible with oxidizers. When heated todecomposition it emits acrid and irritating fumes.
Potential Exposure
Benzophenone is used in UV curing of inks and coatings; as an intermediate; as an odor fixative in fragrances, flavoring, soaps; in the manufacture of pharmaceuticals and insecticides; in organic syntheses.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, rinse mouth and get medical attention.
Chemical properties
Benzophenone is a common photosensitizer in photochemistry. It crosses from the S1 state into the triplet state with nearly 100 % yield. The resulting diradical will abstract a hydrogen atom from a suitable hydrogen donor to form a ketyl radical.
Carcinogenicity
Lifetime dermal carcinogenicity studies in mice and rabbits did not show any tumor excess in the treated animals. Female Swiss mice and New Zealand White rabbits of both sexes were treated dermally with 0, 5, 25, or 50% of benzophenone (0.02 mL) twice a week for 120 or 180 weeks. Weekly examination of the rabbits did not reveal any reduction in survival or appearance of tumors. Mice treated with benzophenone did not show any excess in the number of tumor-bearing animals or in total number of tumors compared to untreated control animals. Although three skin tumors were observed in the benzophenone- treated mice (one case of squamous cell carcinoma and two cases of squamous cell papilloma), there were also three tumors (one carcinoma and toe papillomas) observed in the control animals.
Metabolism
Benzophenone's main metabolic pathway in the rabbit is by reduction to benzhydrol, which is excreted conjugated with glucuronic acid(Williams, 1959).
Preparation
Benzophenone can be prepared by the reaction of benzene with carbon tetrachloride followed by hydrolysis of the resulting diphenyldichloromethane, or by Friedel-Crafts acylation of benzene with benzoyl chloride in the presence of a Lewis acid (e.g. aluminum chloride) catalyst.
Safety
Benzophenone is considered "essentially nontoxic." It is however banned as a food additive by the US Food and Drug Administration, despite the FDA's continuing stance that this chemical does not pose a risk to public health under the conditions of its intended use.
Solubility in organics
Insoluble in water and glycerin, slightly soluble in Propylene glycol, 6% soluble in alcohol, soluble inmost perfume oils.
Storage
Color Code—Green: General storage may be used.Store in a cool, well-ventilated area away from sources ofignition and incompatible materials.
Shipping
UN1224 Ketones, liquid, n.o.s., Hazard Class: 3; Labels: 3—Flammable liquid, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Crystallise it from MeOH, EtOH, cyclohexane, *benzene or pet ether, then dry in a current of warm air and store it over BaO or P2O5. It is also purified by zone melting and by sublimation [Itoh J Phys Chem 89 3949 1985, Naguib et al. J Am Chem Soc 108 128 1986, Gorman & Rodgers J Am Chem Soc 108 5074 1986, Ohamoto & Teranishi J Am Chem Soc 108 6378 1986, Naguib et al. J Phys Chem 91 3033 1987]. [Beilstein 7 III 2048, 7 IV 1357.]
Incompatibilities
Oxidizing materials, such as dichromates and permanganates.
Properties of Benzophenone
| Melting point: | 47-51 °C (lit.) |
| Boiling point: | 305 °C (lit.) |
| Density | 1.11 |
| vapor density | 4.21 (vs air) |
| vapor pressure | 1 mm Hg ( 108 °C) |
| refractive index | 1.5893 |
| FEMA | 2134 | BENZOPHENONE |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | ethanol: soluble100mg/mL, clear, colorless (80% ethanol) |
| form | Crystalline Powder or Flakes |
| color | White to off-white |
| Odor | Characteristic. |
| Water Solubility | insoluble (<0.1 g/100 mL at 25 ºC) |
| Merck | 14,1098 |
| JECFA Number | 831 |
| BRN | 1238185 |
| Dielectric constant | 13.0(20℃) |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong reducing agents. Combustible. |
| CAS DataBase Reference | 119-61-9(CAS DataBase Reference) |
| IARC | 2B (Vol. 101) 2013 |
| NIST Chemistry Reference | Benzophenone(119-61-9) |
| EPA Substance Registry System | Benzophenone (119-61-9) |
Safety information for Benzophenone
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H373:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P314:Get medical advice/attention if you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Benzophenone
| InChIKey | RWCCWEUUXYIKHB-UHFFFAOYSA-N |
Benzophenone manufacturer
Clickchem Research LLP
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
Akhil Healthcare Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![3a,6a-Diphenyloctahydroimidazo[4,5-d]imidazole-2,5-dione](https://img.chemicalbook.in/CAS/GIF/5157-15-3.gif)
You may like
-
 Benzophenone supplierView Details
Benzophenone supplierView Details
119-61-9 -
 Benzophenone 98%View Details
Benzophenone 98%View Details -
 Benzophenone 98%View Details
Benzophenone 98%View Details -
 Benzophenone (SQ) CAS 119-61-9View Details
Benzophenone (SQ) CAS 119-61-9View Details
119-61-9 -
 Benzophenone extrapure CAS 119-61-9View Details
Benzophenone extrapure CAS 119-61-9View Details
119-61-9 -
 Benzophenone ExiPlus CAS 119-61-9View Details
Benzophenone ExiPlus CAS 119-61-9View Details
119-61-9 -
 Benzophenone CAS 119-61-9View Details
Benzophenone CAS 119-61-9View Details
119-61-9 -
 BENZOPHENONE 99% Extra Pure (1000gm)View Details
BENZOPHENONE 99% Extra Pure (1000gm)View Details
119-61-9
