Nilvadipine
Synonym(s):2-Cyano-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-methyl 5-(1-methylethyl) ester;5-Isopropyl-3-methyl-2-cyano-1,4-dihydro-6-methyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate;Isopropyl 6-cyano-5-methoxycarbonyl-2-methyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylate;Nivadipine;Nivaldipine
- CAS NO.:75530-68-6
- Empirical Formula: C19H19N3O6
- Molecular Weight: 385.37
- MDL number: MFCD00867065
- EINECS: 805-887-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
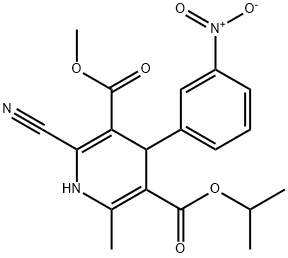
What is Nilvadipine?
Description
Nilvadipine is a new, second-generation calcium channel blocker effective in the treatment of hypertension and angina pectoris. Its potent inhibitory effect on the stimulated chemotaxis of smooth muscle cells and protective action against calcium deposition suggest that nilvadipine may be useful for preventing and treating atherosclerosis.
Chemical properties
Yellow Prisms
Originator
Fujisawa (Japan)
The Uses of Nilvadipine
Used as an antihypertensive and antianginal
The Uses of Nilvadipine
antihypertensive;calcium channel blocker
The Uses of Nilvadipine
Used as an antihypertensive and antianginal.
Background
Nilvadipine is a calcium channel blocker (CCB) for the treatment of hypertension.
Indications
For the management of vasospastic angina, chronic stable angina and hypertension.
What are the applications of Application
Nilvadipine is a potent calcium channel protein inhibitor and blocker
Definition
ChEBI: Nilvadipine is an isopropyl ester, a methyl ester, a nitrile and a dihydropyridine.
Manufacturing Process
To a solution of isopropyl ester of 6-formyl-5-methoxycarbonyl-2-methyl-4-(3- nitrophenyl)-1,4-dihydropyridine- 3-carboxylic acid (4.5 g) in acetic acid (35 ml) were added hydroxylamine hydrochloride (0.97 g) and sodium acetate (1.43 g), and the mixture was stirred at ambient temperature for 2.5 hours. After acetic anhydride (4.14 g) was added to this reaction mixture, the mixture was stirred at ambient temperature for 1.5 hours and at 95-100°C for additional 4 hours. The acetic acid and the excess of acetic anhydride were removed in vacuum, followed by adding water to the residue and it was neutralized with a saturated aqueous solution of sodium bicarbonate. This aqueous suspension was extracted twice with ethyl acetate, and the combined extract was washed with water, dried over anhydrous magnesium sulfate and evaporated to dryness under reduced pressure to give a reddish-brown oil (4.88 g), which was chromatographed over silica gel (150 g) with a mixture of benzene and ethyl acetate (10:1 by volume) as an eluent to give a crude crystals (2.99 g). These were recrystallized from ethanol to give yellow prisms (1.89 g) of isopropyl ester of 6-cyano-5-methoxycarbonyl-2-methyl-4-(3- nitrophenyl)-1,4-dihydropyridine-3-carboxylic acid melting point 148-150°C (yellow prisms from ethanol); [α]D20 = 222.4° (c = 1 in methanol).
brand name
Nivadil
Therapeutic Function
Calcium entry blocker, Antihypertensive
Pharmacokinetics
Nilvadipine is similar to other dihydropyridines including amlodipine, felodipine, isradipine, and nicardipine. Nilvadipine is used to treat Prinzmetal's angina, hypertension, and other vascular disorders such as Raynaud's phenomenon. By blocking the calcium channels, Nifedipine inhibits the spasm of the coronary artery and dilates the systemic arteries, results in a increase of myocardial oxygen supply and a decrease in systemic blood pressure.
Metabolism
Not Available
References
1) Paris?et al. (2014),?The spleen tyrosine kinase (Syk) regulates Alzheimer amyloid-β production and Tau hyperphosphorylation; J. Biol. Chem.,?289?33927 2) Rosenthal (1994),?Nilvadipine: profile of a new calcium antagonist. An overview; J. Cardiovasc. Pharmacol. 24 Suppl 2?S92 3) Nimmrich and Eckert (2013),?Calcium channel blockers and dementia; Br. J. Pharmacol.?169?1203 4) Otori?et al. (2003),?Protective effect of nilvadipine against glutamate neurotoxicity in purified retinal ganglion cells; Brain Res.?961?213
Properties of Nilvadipine
| Melting point: | 148-150°C |
| Boiling point: | 511.83°C (rough estimate) |
| Density | 1.3937 (rough estimate) |
| refractive index | 1.5900 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | DMSO: soluble15mg/mL, clear |
| form | powder |
| pka | -0.81±0.70(Predicted) |
| color | white to beige |
| Merck | 14,6545 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
Safety information for Nilvadipine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Nilvadipine
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE 4-Bromopyrazole 5,6-Dimethoxyindanone Tert-butyl bis(2-chloroethyl)carbamate (2-Hydroxyphenyl)acetonitrile 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Nilvadipine CAS 75530-68-6View Details
Nilvadipine CAS 75530-68-6View Details
75530-68-6 -
 Nilvadipine 99% (HPLC) CAS 75530-68-6View Details
Nilvadipine 99% (HPLC) CAS 75530-68-6View Details
75530-68-6 -
 Nilvadipine 98% (HPLC) CAS 75530-68-6View Details
Nilvadipine 98% (HPLC) CAS 75530-68-6View Details
75530-68-6 -
 Nilvadipine CAS 75530-68-6View Details
Nilvadipine CAS 75530-68-6View Details
75530-68-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1