Nilotinib Hydrochloride Monohydrate
- CAS NO.:923288-90-8
- Empirical Formula: C28H25ClF3N7O2
- Molecular Weight: 583.9920096
- MDL number: MFCD18251359
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
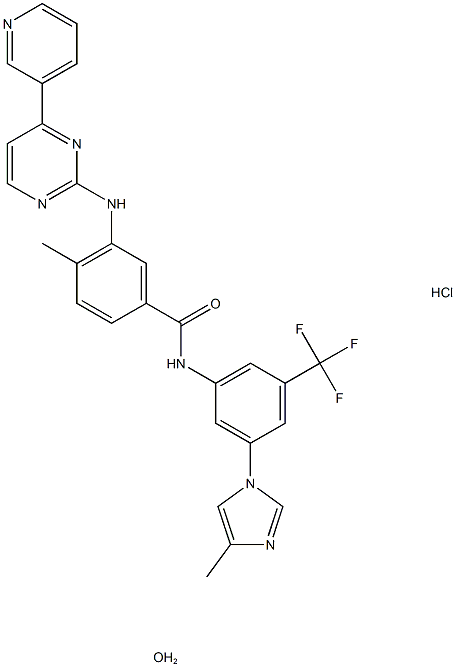
What is Nilotinib Hydrochloride Monohydrate?
Chemical properties
Nilotinib hydrochloride monohydrate (NHM) lacks a chiral center, making it incapable of tautomerism. NHM is a chemical compound with the following name: 4-methyl-N-[3-(4-methyl-1H-imidazol1-yl)-5-(trifuoromethyl) phenyl]-3-[(4-pyridin-3-ylpyrimidin-2-yl) amino] benzamide hydrochloride monohydrate. It is a white powder with a slight yellowish or greenish-yellowish shade. At 25 °C, the aqueous solubility of NHM markedly decreases with pH. Moreover, it is almost insoluble in bufer solutions with pH values higher than 4.5. NHM shows slight solubility in ethanol and methanol. Nilotinib Hydrochloride Monohydrate is the monohydrate monohydrochloride form of nilotinib, an orally bioavailable aminopyrimidine-derivative Bcr-Abl tyrosine kinase inhibitor with antineoplastic activity.
The Uses of Nilotinib Hydrochloride Monohydrate
Nilotinib Monohydrochloride Monohydrate might be useful in treatment of chronic myelogenous leukemia. It is a COVID19-related research product.
Definition
Nilotinib, also known as AMN107, is a tyrosine kinase inhibitor under investigation as a possible treatment for chronic myelogenous leukemia (CML). A Phase I clinical trial in 2006 showed that this drug was relatively safe and offered significant therapeutic benefits in cases of CML which were found to be resistant to treatment with imatinib (Gleevec), another tyrosine kinase inhibitor used as a first-line treatment for CML.
Mechanism of action
Tasigna (nilotinib hydrochloride monohydrate) is an orally available signal transduction inhibitor of the Bcr-Abl kinase, c-kit and Platelet Derived Growth Factor (PDGF). Nilotinib binds to and stabilizes the inactive conformation of the kinase domain of Abl protein. In vitro, nilotinib inhibited Bcr-Abl mediated proliferation of murine leukemic cell lines and human cell lines derived from Ph+ CML patients. In vivo, nilotinib reduced the tumor size in a murine Bcr-Abl xenograft model.
Side Effects
a headache, fatigue, gastrointestinal problems such as nausea, vomiting, diarrhea and constipation, muscle and joint pain, rash and other skin conditions, flu-like symptoms, and reduced blood cell count. Less typical side effects are those of the cardiovascular system, such as hypertension (high blood pressure), various types of arrhythmia, and prolonged QT interval. Interaction of nilotinib with OATP1B1 and OATP1B3 may alter its hepatic disposition and can lead to transporter mediated drug-drug interactions.
in vitro
In vitro, nilotinib inhibited Bcr-Abl mediated proliferation of murine leukemic cell lines and human cell lines derived from Ph+ CML patients. Under the conditions of the assays, nilotinib was able to overcome imatinib resistance resulting from Bcr-Abl kinase mutations, in 32 out of 33 mutations tested. In vivo, nilotinib reduced the tumor size in a murine Bcr-Abl xenograft model. Nilotinib inhibited the autophosphorylation of the following kinases at IC50 values as indicated: Bcr-Abl (20-60 nM), PDGFR (69 nM) and c-Kit (210 nM). Nilotinib is currently being trialed in people with Parkinson's disease, as it appears to be able to halt progression of the disease and even improve their symptoms.
Properties of Nilotinib Hydrochloride Monohydrate
| Melting point: | 198-201°C |
| storage temp. | -20°C Freezer |
| solubility | Aqueous Acid (Slightly), DMSO (Slightly), Ethanol (Slightly, Heated) |
| form | Solid |
| color | Off-White to Pale Beige |
Safety information for Nilotinib Hydrochloride Monohydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Nilotinib Hydrochloride Monohydrate
| InChIKey | YCBPQSYLYYBPDW-UHFFFAOYSA-N |
| SMILES | N1(C=C(N=C1)C)C1C=C(C=C(C=1)NC(=O)C1C=CC(=C(NC2=NC=CC(C3=CC=CN=C3)=N2)C=1)C)C(F)(F)F.Cl.O |
Nilotinib Hydrochloride Monohydrate manufacturer
Archerchem Healthcare Pvt., Ltd. (part of Archerchem Group)
New Products
3-pyridine carboxaldehyde 7-bromoquinoxalin-2(1H)-one 2-Bromo-6-flurobenzaldehyde 8-Bromoisoquinoline 3-Pyridineacetonitrile, α-amino-, hydrochloride (1:1) Ethyl 3-Phenoxypropionate 4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE TosMIC 2-Iodo-5-methoxybenzoic acid Xanomeline Suzetrigine Remibrutinib Dimethyl 4-aminothiophene-2,3-dicarboxylate hydrochloride 1H-Indole-1-carboxylic acid, 5-hydroxy-7-methyl-, 1,1-dimethylethyl ester N N N'Trimethyl ethylenediamine Lead II Bromide Variamine Blue B Diazonium salt N N' DimethylEthylenediamine Ethyl Methanesulfonate N Ethylmethylamine Radiator Flux Zinc Chloride Solution (All Grades) Calcium Alphaketoglutarate* Fmoc-L-Tyr(tBu)-OHRelated products of tetrahydrofuran
![4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid](https://img.chemicalbook.in/CAS/GIF/641569-94-0.gif)
You may like
-
 923288-90-8 Nilotinib Hydrochloride Monohydrate (Form B) 98%View Details
923288-90-8 Nilotinib Hydrochloride Monohydrate (Form B) 98%View Details
923288-90-8 -
 923288-90-8 98%View Details
923288-90-8 98%View Details
923288-90-8 -
 Nilotinib Hydrochloride Monohydrate 99%View Details
Nilotinib Hydrochloride Monohydrate 99%View Details
923288-90-8 -
 Nilotinib monohydrochloride monohydrate 99% (HPLC) CAS 923288-90-8View Details
Nilotinib monohydrochloride monohydrate 99% (HPLC) CAS 923288-90-8View Details
923288-90-8 -
 Nilotinib CAS 923288-90-8View Details
Nilotinib CAS 923288-90-8View Details
923288-90-8 -
 1181267-33-3 99%View Details
1181267-33-3 99%View Details
1181267-33-3 -
 3-Amino-4-pyrazole carboxamide hemisulfate 27511-79-1 99%View Details
3-Amino-4-pyrazole carboxamide hemisulfate 27511-79-1 99%View Details
27511-79-1 -
 3-Dimethl amino-1- (naphthalen-1-yl)propan-1- one hydro chloride 99%View Details
3-Dimethl amino-1- (naphthalen-1-yl)propan-1- one hydro chloride 99%View Details
5409-58-5
