Nickelous acetate
- CAS NO.:373-02-4
- Empirical Formula: C4H6NiO4
- Molecular Weight: 176.78
- EINECS: 206-761-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
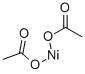
What is Nickelous acetate?
Description
Nickel (II) acetate (Ni (CH3 COO)2 ) is an inorganic compound of nickel and acetic acid. This inorganic compound is usually found as the tetrahydrate. It is used for electroplating.
It can be made by reacting nickel or nickel (II) carbonate with acetic acid.
Ni + 2 CH3 COOH → C4 H6 NiO4 + H2
NiCO3 + 2 CH3 COOH → C4 H6 NiO4 + CO2 + H2 O
The green tetrahydrate has been determined by X-ray crystallography to be octahedral about the central nickel atom, coordinated by four water molecules and two acetate fragments.It may be dehydrated in vacuo, by reaction with acetic anhydride,or by heat.
Chemical properties
Green, monoclinic crystals; effloresces somewhat in air.Soluble in water and alcohol.
Chemical properties
Nickel(II) acetate crystallizes from solutions of the hydroxide in acetic acid at room temperature as the green tetrahydrate. This is isomorphous with the cobalt(II) salt having a distorted octahedral structure with the nickel atoms surrounded by four water molecules and two oxygens from two acetato groups which are trans to each other. Its magnetic moment is 3.30 BM at room temperature. The hygroscopic anhydrous acetate is obtained by dehydration of the tetrahydrate in vacuo or by heating the tetrahydrate under reflux with acetic anhydride. Like the tetrahydrate it is magnetically dilute at room temperature. Upon thermal decomposition in nitrogen (or in vacuo) at 300° it evolves acetone, acetic acid, carbon monoxide, carbon dioxide and water leaving nickel and N13C.
Physical properties
The tetrahydrate is a green crystalline solid; sweet taste; odor of acetic acid; density 1.744 g/cm3; loses water on heating to form a yellow-green powder of anhydrous nickel acetate; decomposes above 250°C; soluble in water, 17g/100mL at 20°C; sparingly soluble in alcohol.
The Uses of Nickelous acetate
Textiles (mordant), catalyst.
General Description
Dull green odorless solid. Sinks and mixes slowly with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Nickelous acetate is a green, crystalline material, mildly toxic and carcinogenic. Combustible when exposed to heat or flame. When heated to decomposition Nickelous acetate emits acrid smoke and irritating fumes [Lewis, 3rd ed., 1993, p. 909].
Hazard
Toxic by ingestion, a carcinogen (OSHA).
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion causes vomiting. Contact with eyes causes irritation. May cause dermatitis in contact with skin.
Flammability and Explosibility
Non flammable
Safety Profile
Confirmed carcinogen with experimental neoplastigenic and tumorigenic data. Poison by ingestion, intraperitoneal, and subcutaneous routes. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits irritating fumes. See also NICKEL COMPOUNDS.
Safety
Nickel salts are carcinogenic and irritate the skin.
Properties of Nickelous acetate
| Melting point: | 1555°C |
| Boiling point: | 16.6°C |
| Density | 1.798 g/cm3 |
| form | solid |
| Water Solubility | 177g/L at 20℃ |
| EPA Substance Registry System | Nickel(II) acetate (373-02-4) |
Safety information for Nickelous acetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Nickelous acetate
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1