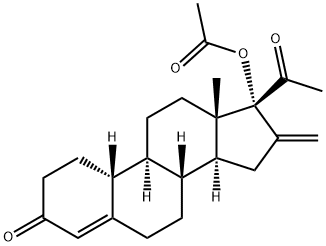
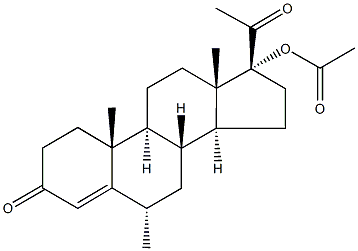
Nestoron
Synonym(s):17-(Acetyloxy)-16-methylene-19-norpregn-4-ene-3,20-dione;17-Hydroxy-16-methylene-19-norpregn-4-ene-3,20-dione acetate;Elcometrine;Nestoron;
- CAS NO.:7759-35-5
- Empirical Formula: C23H30O4
- Molecular Weight: 370.48
- MDL number: MFCD01711303
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-14 14:09:35
What is Nestoron?
Absorption
Contraceptive vaginal rings provided sustained release of contraceptive levels of segesterone acetate over 90 days in a pharmacokinetic study of healthy women . Following vaginal administration for up to 13 cycles, segesterone acetate was absorbed into systemic administration and reached the peak plasma concentration in 2 hours in Cycle 1, Cycle 3, and Cycle 13. Concentrations declined after time to reach plasma concentration (Tmax) and became more constant after 96 hours post-dose.Over subsequent cycles of use, the peak serum concentrations of segesterone acetate decreased. In Cycle 1, 3 and 13, the peak plasma concentrations were 1147, 363, and 294 pg/mL .
Toxicity
There have been no reports of serious ill effects from overdose of combination hormonal contraceptive use. Overdosage may cause withdrawal bleeding in females and nausea. In case of suspected overdose, all vaginal systems containing segesterone acetate should be removed and symptomatic treatment should be initiated .
In a 2-year carcinogenicity study in rats receiving segesterone acetate via subdermal implants, there was no drug-related increase in tumor incidence. In a 2-year intravaginal carcinogenicity study in mice, segesterone acetate gel produced an increased incidence of adenocarcinoma and lobular hyperplasia in the breast at a supratherapeutic dose of 30 mg/kg/day. Segesterone acetate was not shown to be mutagenic or clastogenic .
The Uses of Nestoron
Segesterone Acetate has been used to study the: comparison of androgenic and estrogenic properties of progestins used in contraception and hormone therapy; impact on hepatic estrogen-sensitive proteins by 1-yr contraceptive vaginal ring delivering Nestorone and ethinyl estradiol.
Indications
Segesterone acetate in combination with ethinyl estradiol is indicated for use by females of reproductive potential to prevent pregnancy as a combination hormonal contraceptive (CHC). It induces contraception for thirteen 28-day cycles (1 year) following vaginal administration. The vaginal system must remain in place continuously for 3 weeks (21 days) followed by a 1-week (7-day) vaginal system-free interval. The use in females with a body mass index of >29 kg/m^2 has not been adequately evaluated .
Background
Segesterone acetate is a steroidal progestin or synthetic progesterone and a 19-norprogesterone derivative with no CH3 group radical in position 6 . In animal studies, segesterone acetate was shown to be one of the most potent progestins . It mediates progestational activity 100 times higher than that of progesterone . It is commonly sold under the brand names Nestorone and Elcometrine and serves as an active component in hormonal contraceptives. It is also used as a treatment for endometriosis in South American countries. Segesterone acetate binds selectively to progesterone receptors and not androgen receptors . Due to its rapid hepatic metabolism, segesterone acetate must be administered parenterally . Segesterone acetate is not an orally active compound, but it is proved to be a potent anti-ovulatory agent when given in implants, vaginal rings or percutaneous gel .
On August 10, 2018, Annovera? containing segesterone acetate and ethinyl estradiol was granted approval by the U.S. Food and Drug Administration (FDA) as the first and only contraceptive that provides an entire year of protection against unintended pregnancy while entirely under a woman's control. According to the Center for Disease Control, more than 43 million women in the U.S. are at risk of unintended pregnancy, which may be associated with an elevated risk for improper prenatal care, premature and low-birth-weight infants, and physical and mental health risks . The introduction of this new contraceptive method offers an expansion of birth control options for women while maintaining high efficacy and acceptability similar to existing shorter-acting combined hormonal methods . In clinical trials, Annovera? achieved a 97.3% success in pregnancy prevention . Annovera? is administered as a vaginal ring that is in place for 21 days and removed for 7 days each cycle. As with other hormonal contraceptives, Annovera? carries the risk for serious cardiovascular events.
Definition
ChEBI: Elcometrine is a corticosteroid hormone.
Pharmacokinetics
Segesterone acetate suppresses ovulation. In a Phase I randomized, placebo-controlled, randomized crossover study involving healthy adult female subjects, there was no clinically significant QTc interval prolongation following a single intravenous bolus dose of segesterone acetate . Segesterone acetate shows no androgenic, anabolic, or estrogenic activity . It also did not show uterotropic activity in ovariectomized rats . In the endometrial transformation test to assess the progestational activity, dose-dependent increases in both uterine weight was observed following subcutaneous administration of segesterone acetate .
Pharmacokinetics
Elcometrine is a member of the 19-norprogesterone class of pr ogestins. when administered subcuta neously (implant), intravaginally (vaginal ring), or transdermally, elcometrine is an exceptionally potent progestin. It is 100-fold more potent when administered subcutaneously than when delivered orally. Its oral bioavaiability is only 10%,with a half life of just 1 to 2 hours because of rapid metabolism. The C16 methylene functionality substantially increases its affinity for the progesterone receptor.
Clinical Use
Because of its antiestrogenic action, elcometrine also can be administered transdermally along with estradiol as a treatment for menopausal symptoms .
Metabolism
Segesterone acetate undergoes rapid metabolism and inactivation in the liver . Based on the findings in vitro, the major oxidative metabolites in the serum include 5α-dihydro- and 17α-hydroxy-5α--dihydro metabolites constitute about 50% of exposure relative to segesterone acetate. The metabolites are not pharmacologically active with EC50 to progesterone receptor 10-fold higher than that of the parent compound . It was shown that 3α, 5α-tetrahydrosegesterone acetate acts as an activator at the GABA-A receptors in the brain .
Properties of Nestoron
| Melting point: | 178-179° |
| Boiling point: | 420.37°C (rough estimate) |
| alpha | D21 -105° (c = 1.2 in chloroform) |
| Density | 1.0550 (rough estimate) |
| refractive index | 1.4433 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble5mg/mL, clear |
| form | powder |
| color | white to beige |
Safety information for Nestoron
Computed Descriptors for Nestoron
| InChIKey | CKFBRGLGTWAVLG-GOMYTPFNSA-N |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran



You may like
-
 Nestorone CAS 7759-35-5View Details
Nestorone CAS 7759-35-5View Details
7759-35-5 -
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0