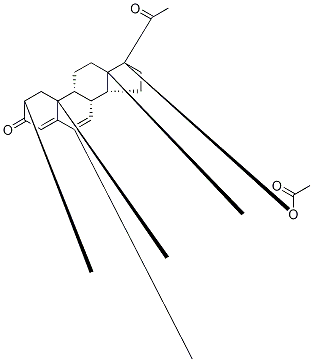
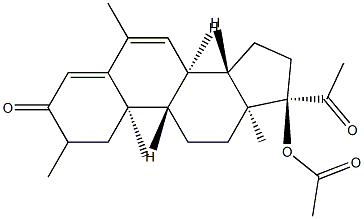
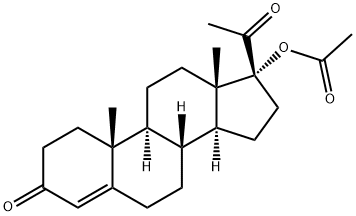
Megestrol acetate
Synonym(s):Megestrol acetate;Megestrol 17-acetate;17α-Acetoxy-6-methyl-4,6-pregnadiene-3,20-dione;17α-Hydroxy-6-methyl-4,6-pregnadiene-3,20-dione 17-acetate;17α-Hydroxy-6-methyl-6-dehydroprogesterone 17-acetate
- CAS NO.:595-33-5
- Empirical Formula: C24H32O4
- Molecular Weight: 384.51
- MDL number: MFCD00056470
- EINECS: 209-864-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
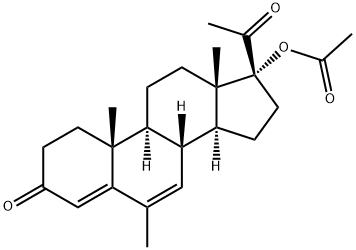
What is Megestrol acetate ?
Absorption
Variable, but well absorbed orally.
Toxicity
No serious unexpected side effects have resulted from studies involving megestrol acetate oral suspension administered in dosages as high as 1200 mg/day. Treatment with megestrol acetate, an orexigenic agent, has also resulted in iatrogenic adrenal suppression. The mechanism is presumably related to the glucocorticoid properties of megestrol acetate [PMID: 12872362].
Description
Megestrol Acetate is the acetate salt form of megestrol, a synthetic derivative of the naturally occurring female sex hormone progesterone with potential anti-estrogenic and antineoplastic activity. It is frequently used in the treatment of patients with metastatic breast cancer. It is generally well tolerated, except that it may cause undesirable weight gain. Subsequently, it was shown that megestrol acetate produced weight gain in a variety of cachectic cancer patients. Significant reduction inserum levels of IL-1a and b,IL-2,IL-6, and TNF-o were observed in cancer patientstreated with megestrol acetate which may bear on the mechanism of improved appetite and body weight gain.It has also been postulated that the effect is, at least in part,mediated by NPY,a potent central appetite stimulant.
Chemical properties
Crystalline Solid
Originator
Megestat,Bristol,W. Germany,1964
The Uses of Megestrol acetate
Megestrol acetate (Megace), a semi-synthetic progestational steroid, is the most effective known appetite stimulant at present. Previously used in patients with cancer and acquired immunodeficiency syndrome, only a few studies have considered the applicability of this drug in malnourished older persons.
Megestrol acetate is useful if profound anorexia is the main manifestation of cachexia and if expected survival is weeks to months. Megace has been shown to significantly increase appetite, but not survival. In patients with a shorter predicted survival, a brief course of corticosteroids may be useful to stimulate appetite and have positive effects on nausea, pain, and asthenia.
The Uses of Megestrol acetate
Megestrol acetate USP (Megace) is used to treat Carcinoma of the breast or endorometrium.
Indications
For the treatment of anorexia, cachexia, or an unexplained, significant weight loss in patients with a diagnosis of acquired immunodeficiency syndrome (AIDS). Also used for the palliative management of recurrent, inoperable, or metastatic breast cancer, endometrial cancer, and prostate cancer in Canada and some other countries.
Background
17-Hydroxy-6-methylpregna-3,6-diene-3,20-dione. A progestational hormone used most commonly as the acetate ester. As the acetate, it is more potent than progesterone both as a progestagen and as an ovulation inhibitor. It has also been used in the palliative treatment of breast cancer.
What are the applications of Application
Megestrol Acetate is a progesterone derivative with antineoplastic properties
Definition
ChEBI: Megestrol acetate is a steroid ester resulting from the formal condensation of the hydroxy group of megestrol with the carboxy group of acetic acid. It is an appetite stimulant used for the treatment of anorexia and cachexia. Also used for birth control and for the treatment of breast cancer. It has a role as an antineoplastic agent, an appetite enhancer, a contraceptive drug, a progestin and a synthetic oral contraceptive. It is a steroid ester, an acetate ester, a 20-oxo steroid and a 3-oxo-Delta(4) steroid. It derives from a megestrol.
Manufacturing Process
The following preparation is given in US Patent 3,356,573. 17α-Acetoxy-3βhydroxy-6-methylpregn-5-ene-20-one (1 g), aluminum tert-butoxide (1 g) and p-benzoquinone (6 g) were dissolved in dry benzene (100 ml) and the mixture was heated under reflux for 30 minutes. The reaction mixture was cooled and washed with potassium hydroxide solution until the benzene layer was colorless. The benzene was washed with water, dried and evaporated to dryness under reduced pressure. The residue crystallized from aqueous methanol to give 17α-acetoxy-6-methylpregna-4,6-diene-3,20-dione, needles, MP 214° to 216°C.
brand name
Megace (Bristol-Myers Squibb); Megace (Par).
Therapeutic Function
Cancer chemotherapy
General Description
Megestrol acetate, 17-hydroxy-6-methylpregna-4,6-diene-3,20-dione acetate(Megace), is a progestin used primarily for the palliativemanagement of recurrent, inoperable, or metastatic endometrialor breast carcinoma. Megestrol acetate has also beenindicated for appetite enhancement in patients with AIDS.The biochemical basis for this use of megestrol is unclear.
Pharmacokinetics
Megestrol is a synthetic progestin and has the same physiologic effects as natural progesterone. These effects include induction of secretory changes in the endometrium, increase in basal body temperature, pituitary inhibition, and production of withdrawal bleeding in the presence of estrogen. Mestrogel has slight glucocorticoid activity and very slight mineralocorticoid activity. This drug has no estrogenic, androgenic, or anabolic activity. The precise mechanism of megestrol’s antianorexic and anticachetic effects is unknown. Initially developed as a contraceptive, it was first evaluated in breast cancer treatment in 1967.
Clinical Use
Progestin activity is further enhanced when a double bond is introduced between positions 6 and 7, as is found in megestrol acetate. Megestrol is used primarily in the treatment of breast and endometrial carcinomas and in postmenopausal women with advanced hormone-dependent carcinoma.
Safety Profile
Suspected carcinogen with experimental carcinogenic and teratogenic data. Poison by intravenous route. Human reproductive effects bp ingestion and implant routes: effects on ovaries and fallopian tubes, menstrual cycle changes, and female fertility index changes. Mutation data reported. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes. An FDA proprietary drug used to treat endometriosis and breast cancer. A steroid.
Veterinary Drugs and Treatments
Megestrol acetate (Ovaban?—Schering) is approved by FDA for use
in dogs only for the postponement of estrus and the alleviation of
false pregnancy. In male dogs, it has been used for benign prostatic
hypertrophy. It is used clinically for many dermatologic and behavior-
related conditions, primarily in the cat. See the Dosage section
for specific indications and dosages for both dogs and cats.
Megestrol acetate is indicated in humans for the palliative treatment
of advanced carcinoma of the breast or endometrium.
Metabolism
Primarily hepatic. Megestrol metabolites which were identified in urine constituted 5% to 8% of the dose administered. Respiratory excretion as labeled carbon dioxide and fat storage may have accounted for at least part of the radioactivity not found in urine and feces. No active metabolites have been identified.
Metabolism
Less than 10% of an oral dose undergoes metabolism. Several major metabolites appear in the urine (e.g., 2-hydroxy and 6-hydroxymethyl megestrol and their glucuronide conjugates).
Storage
Store at RT
References
[1] zhang k1, chow pk. the effect of megestrol acetate on growth of hepg2 cells in vitro and in vivo. clin cancer res. 2004 aug 1; 10(15):5226-32.
Properties of Megestrol acetate
| Melting point: | 214°C |
| Boiling point: | 431.17°C (rough estimate) |
| alpha | D24 +5° (chloroform) |
| Density | 1.0474 (rough estimate) |
| refractive index | 11 ° (C=2, CHCl3) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Practically insoluble in water, soluble in acetone, sparingly soluble in alcohol. |
| form | neat |
| form | Solid |
| color | White |
| Merck | 14,5805 |
| BRN | 1917291 |
| CAS DataBase Reference | 595-33-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Megestrol acetate(595-33-5) |
| EPA Substance Registry System | Megestrol acetate (595-33-5) |
Safety information for Megestrol acetate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Megestrol acetate
| InChIKey | RQZAXGRLVPAYTJ-GQFGMJRRSA-N |
| SMILES | C1(=O)C=C2[C@](C)(CC1)[C@]1([H])[C@]([H])([C@@]3([H])[C@@](CC1)(C)[C@@](OC(C)=O)(C(=O)C)CC3)C=C2C |
Megestrol acetate manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 595-33-5 Megestrol acetate 98%View Details
595-33-5 Megestrol acetate 98%View Details
595-33-5 -
 595-33-5 98%View Details
595-33-5 98%View Details
595-33-5 -
 595-33-5 Megestrol Acetate 95.8View Details
595-33-5 Megestrol Acetate 95.8View Details
595-33-5 -
 Megestrol Acetate CAS 595-33-5View Details
Megestrol Acetate CAS 595-33-5View Details
595-33-5 -
 Megestrol acetate 98% (HPLC) CAS 595-33-5View Details
Megestrol acetate 98% (HPLC) CAS 595-33-5View Details
595-33-5 -
 Megestrol acetate CAS 595-33-5View Details
Megestrol acetate CAS 595-33-5View Details
595-33-5 -
 595-33-5 90 % AboveView Details
595-33-5 90 % AboveView Details
595-33-5 -
 Megestrol Acetate Api Powder, Greater Than 99%View Details
Megestrol Acetate Api Powder, Greater Than 99%View Details
595-33-5
