NELFINAVIR
- CAS NO.:159989-64-7
- Empirical Formula: C32H45N3O4S
- Molecular Weight: 567.78
- MDL number: MFCD01938163
- EINECS: 1533716-785-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is NELFINAVIR?
Absorption
Well absorbed following oral administration.
Toxicity
Carcinogenicity studies in mice and rats were conducted with nelfinavir at oral doses up to 1000 mg/kg/day. No evidence of a
tumorigenic effect was noted in mice at systemic exposures (Cmax) up to 9-fold those measured in humans at the recommended
therapeutic dose (750 mg TID or 1250 mg BID). In rats, thyroid follicular cell adenomas and carcinomas were increased in males at
300 mg/kg/day and higher and in females at 1000 mg/kg/day. Systemic exposures (Cmax) at 300 and 1000 mg/kg/day were 1- to 3-fold, respectively, those measured in humans at the recommended therapeutic dose. Repeated administration of nelfinavir to rats produced
effects consistent with hepatic microsomal enzyme induction and increased thyroid hormone deposition; these effects predispose rats, but not humans, to thyroid follicular cell neoplasms. Nelfinavir showed no evidence of mutagenic or clastogenic activity in a battery of in vitro and in vivo genetic toxicology assays. These studies included bacterial mutation assays in S. typhimurium and E. coli, a mouse lymphoma tyrosine kinase assay, a chromosomal aberration assay in human lymphocytes, and an in vivo mouse bone marrow micronucleus assay.
Nelfinavir produced no effects on either male or female mating and fertility or embryo survival in rats at systemic exposures
comparable to the human therapeutic exposure.
Human experience of acute overdose with nelfinavir is limited. There is no specific antidote for overdose with VIRACEPT. If
indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage. Administration of activated charcoal may
also be used to aid the removal of unabsorbed drug. Since nelfinavir is highly protein-bound, dialysis is unlikely to significantly remove the drug from blood.
The Uses of NELFINAVIR
Antiviral.
Background
Nelfinavir is a potent HIV-1 protease inhibitor. It is used in combination with other antiviral drugs in the treatment of HIV in both adults and children. Nelfinavir inhibits the HIV viral proteinase enzyme which prevents cleavage of the gag-pol polyprotein, resulting in noninfectious, immature viral particles.
Indications
Used in combination with other antiviral drugs in the treatment of HIV in both adults and children.
Definition
ChEBI: An aryl sulfide that is used (as its mesylate salt) for treatment of HIV and also exhibits some anticancer properties.
Indications
Nelfinavir (Viracept) is probably the most commonly used protease inhibitor because of its low incidence of serious adverse effects. Its most common side effects are diarrhea and flatulence; these may resolve with continued use. In addition to the drugs contraindicated for use with all protease inhibitors, amiodarone, rifampin, and quinidine are contraindicated in patients taking nelfinavir.
brand name
Viracept (Agouron).
Antimicrobial activity
Nelfinavir inhibits HIV-1 and HIV-2 proteases. Bioavailability is affected to only a limited degree by combination with lowdose ritonavir.
Acquired resistance
Resistance is most frequently selected through a D30N mutation in the HIV protease. An L90M mutation also confers resistance.
Pharmaceutical Applications
A synthetic chemical formulated as the mesylate for oral administration.
Pharmacokinetics
Nelfinavir is a protease inhibitor with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Protease inhibitors block the part of HIV called protease. HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Nelfinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Protease inhibitors are almost always used in combination with at least two other anti-HIV drugs.
Pharmacokinetics
Oral absorption: c. 70–80% (with food)
Cmax 750 mg thrice daily: c. 3–4 mg/L
1250 mg twice daily: c. 4 mg/L
Cmin 750 mg thrice daily: c. 1–3 mg/L
1250 mg twice daily: c. 0.7–2.2 mg/L
Plasma half-life: c. 3.5 h
Volume of distribution: c. 2–7 L/kg
Plasma protein binding: >98%
Absorption and distribution
Food improves the bioavailability and the drug should be administered with a light meal. The semen:plasma ratio is 0.07. It is distributed into breast milk.
Metabolism and excretion
One major and several minor oxidative metabolites are found in plasma. Most of an oral dose is recovered in feces as unchanged drug (22%) and metabolites (78%). The remainder is recovered in urine, mainly unchanged.
An increase in the area under the time–concentration curve (AUC) has been observed in patients with hepatic impairment, but specific dose recommendations have not been made.
Clinical Use
Treatment of HIV infection (in combination with other antiretroviral drugs)
Side Effects
The most common adverse effect is diarrhea of mild to moderate severity. Other side effects include nausea, fatigue, vomiting and headache. It is associated with less dyslipidemia in comparison with ritonavir-boosted protease inhibitors.
Metabolism
Unchanged nelfinavir comprised 82-86% of the total plasma radioactivity after a single oral 750 mg dose of 14C-nelfinavir. In vitro, multiple cytochrome P-450 enzymes including CYP3A and CYP2C19 are responsible for the metabolism of nelfinavir. One major and several minor oxidative metabolites were found in plasma. The major oxidative metabolite has in vitro antiviral activity comparable to the parent drug.
Metabolism
Following oral administration, nelfinavir peak levels in plasma ranged from 0.34 mg/mL (10 mg/kg in the dog) to 1.7 mg/mL (50 mg/kg in the rat). In the dog, nelfinavir was slowly absorbed, and bioavailability was 47%. The drug appeared to be metabolized in the liver, and the major excretory route was in feces.
Properties of NELFINAVIR
| Melting point: | 185-186 °C |
| Boiling point: | 786.8±60.0 °C(Predicted) |
| alpha | D -119.23° (c = 0.26 in methanol) |
| Density | 1.22±0.1 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | ≥ 20.45mg/mL in Ethanol |
| form | Powder |
| pka | pKa1 6.0; pKa2 11.06(at 25℃) |
| color | White to off-white |
| Water Solubility | 7g/L(temperature not stated) |
Safety information for NELFINAVIR
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. |
Computed Descriptors for NELFINAVIR
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


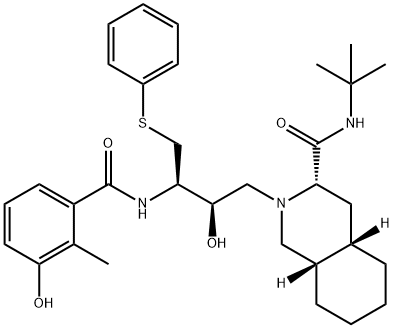
![Nelfinavir Related Compound A (15 mg) ((3S,4aS,8aS)-N-tert-Butyl-2-[(2R,3R)-2-hydroxy-3-(3-hydroxy-2-methylbenzamido)-4-(phenylsulfinyl)butyl]decahydroisoquinoline-3-carboxamide)](https://img.chemicalbook.in/CAS/GIF/1041389-28-9.gif)
![(3S,4aS,8aS)-N-(1,1-DiMethylethyl)decahydro-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-Methylbenzoyl)aMino]-4-(phenylsulfonyl)butyl]-3-isoquinolinecarboxaMide](https://img.chemicalbook.in/CAS/GIF/1041389-29-0.gif)


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1