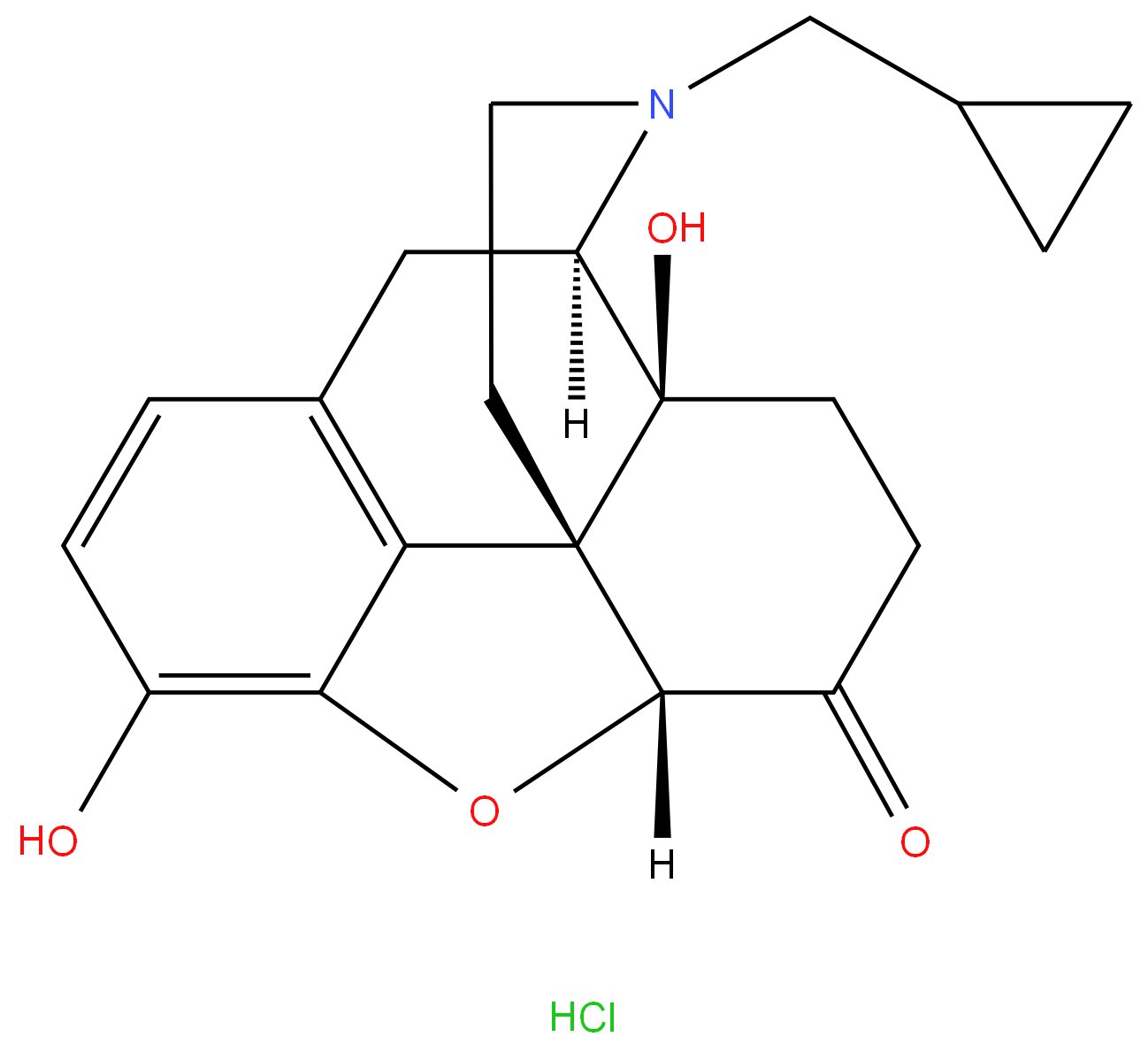
Naltrexone hydrochloride
Synonym(s):Naltrexone HCl
- CAS NO.:16676-29-2
- Empirical Formula: C20H24ClNO4
- Molecular Weight: 377.87
- MDL number: MFCD00069324
- EINECS: 240-723-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
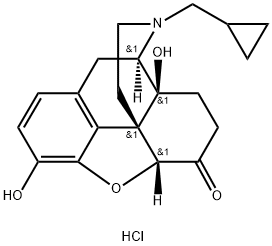
What is Naltrexone hydrochloride?
Description
Naltrexone hydrochloride is a potent, long-acting, orally-effective narcotic antagonist useful in the management of narcotic addiction.
Chemical properties
White Crystalline Powder
Originator
Endo (USA)
The Uses of Naltrexone hydrochloride
Naltrexone hydrochloride has been used:
as an opioid antagonist, to analyse its effect on ethanol preference using Caenorhabditis elegans as a model.
to determine its effectiveness in reducing the preference for substance of abuse (SOA) like nicotine and cocaine using Caenorhabditis elegans as a model.
in the preparation of combinatorial drug, PXT3003 for treating Charcot-Marie-Tooth disease 1A (CMT1A) transgenic rat model Pmp22.
The Uses of Naltrexone hydrochloride
Nonselective opioid receptor antagonist; congener of naloxone
The Uses of Naltrexone hydrochloride
sulfonamide, carbonic anhydrase inhibitor, anti-glaucoma agent
The Uses of Naltrexone hydrochloride
Narcotic antagonist, In treatment of?alcoholism
Definition
ChEBI: Naltrexone hydrochloride is a hydrochloride obtained by reaction of oxycodone with one molar equivalent of hydrochloric acid. it is a mu-opioid receptor antagonist that is used to treat alcohol dependence. It has a role as a mu-opioid receptor antagonist, an antidote to opioid poisoning and a central nervous system depressant. It contains a naltrexone(1+).
brand name
TREXAN
Biological Activity
Opioid antagonist.
Biochem/physiol Actions
Competitive antagonist for μ, κ, δ, and σ-opioid receptors; has greater oral efficacy and longer duration of action than naloxone.
Clinical Use
Opioid antagonist:
Adjunctive prophylactic treatment in patient’s
previously opioid dependant
Treatment of alcohol dependence
Drug interactions
Potentially hazardous interactions with other drugs
Opioids: Avoid concomitant use.
Metabolism
Naltrexone is well absorbed from the gastrointestinal tract but is subject to considerable first-pass metabolism and may undergo enterohepatic recycling. It is extensively metabolised in the liver and the major metabolite, 6-β-naltrexol, may also possess weak opioid antagonist activity. It is excreted mainly in the urine, <5% is excreted in the faeces. The renal clearance for naltrexone ranges from 30-127 mL/min and suggests that renal elimination is primarily by glomerular filtration
storage
Room temperature
Purification Methods
This narcotic antagonist has been purified by recrystallisation from MeOH and dried in air. The free base has m 168-170o after recrystallisation from Me2CO. [Cone et al. J Pharm Sci 64 618 1975, Gold et al. Med Res Rev 2 211 1982.]
Properties of Naltrexone hydrochloride
| Melting point: | 274-2760C |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL, clear, colorless |
| form | neat |
| color | White to Off-White |
| Water Solubility | Soluble in water at 50mg/ml. |
| Sensitive | Light Sensitive |
| Merck | 13,6389 |
| BRN | 3580333 |
| CAS DataBase Reference | 16676-29-2(CAS DataBase Reference) |
Safety information for Naltrexone hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Naltrexone hydrochloride
Naltrexone hydrochloride manufacturer
AKASH PHARMA EXPORTS
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran
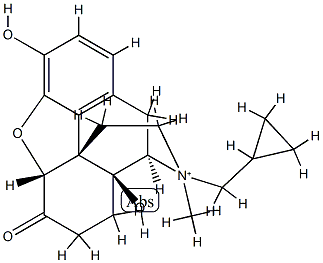
![1-[(2-Chlorophenyl)-N-(methylimino)methyl]cyclopentanol hydrochloride](https://img.chemicalbook.in/CAS/GIF/90717-16-1.gif)
You may like
-
 16676-29-2 99%View Details
16676-29-2 99%View Details
16676-29-2 -
 Naltrexone hydrochloride 16676-29-2 98%View Details
Naltrexone hydrochloride 16676-29-2 98%View Details
16676-29-2 -
 Naltrexone Hydrochloride 99%View Details
Naltrexone Hydrochloride 99%View Details -
 Naltrexone hydrochloride 98%View Details
Naltrexone hydrochloride 98%View Details
16676-29-2 -
 Naltrexone hydrochloride 99%View Details
Naltrexone hydrochloride 99%View Details
16676-29-2 -
 16676-29-2 98%View Details
16676-29-2 98%View Details
16676-29-2 -
 Naltrexone Hydrochloride CAS 16676-29-2View Details
Naltrexone Hydrochloride CAS 16676-29-2View Details
16676-29-2 -
 Naltrexone hydrochloride CAS 16676-29-2View Details
Naltrexone hydrochloride CAS 16676-29-2View Details
16676-29-2
