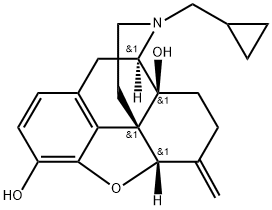
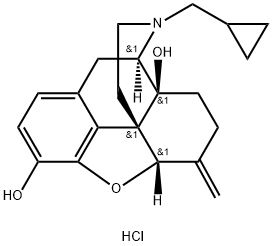
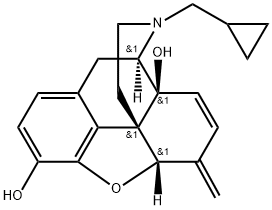
Nalmefene
Synonym(s):(5α)-17-(Cyclopropylmethyl)-4,5-epoxy-6-methylenemorphinan-3,14-diol
- CAS NO.:55096-26-9
- Empirical Formula: C21H25NO3
- Molecular Weight: 339.44
- MDL number: MFCD00133650
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-19 15:37:51
What is Nalmefene?
Absorption
Nalmefene is rapidly absorbed after a single oral administration of 18.06 mg with an absolute oral bioavailability of 41%. The Cmax was 16.5 ng/mL and Tmax was approximately 1.5 hours. The exposure (AUC) was 131 ng x h/mL. High-fat meal increased the AUC by 30% and Cmax by 50% and delayed Tmax by 30 min; however, this is unlikely of clinical significance.
Nalmefene exhibits dose-proportional pharmacokinetics following intravenous administration of 0.5 mg to 2 mg. Tmax was 2.3 ± 1.1 hours following intramuscular administration and 1.5 ± 1.2 hours following subcutaneous administration. Therapeutic plasma concentrations are likely to be reached within 5 to 15 minutes after a 1 mg dose in an emergency. There is variability in the speed of absorption for intramuscular and subcutaneous dosing.
Toxicity
The oral LD50 values were 230 and <200 mg/kg for male and female mice, <300 and 150 mg/kg for male and female rats, and <225 and 225 for male and female rabbits. In mice, rats, and rabbits, intravenous LD50 values had a range of 15.0-48.5 mg/kg and subcutaneous LD50 values were in the range of 157-1150 mg/kg.
Nalmefene was well tolerated and showed no serious toxicity during experimental administration to healthy individuals, even when given at 15 times the highest recommended dose. In a small number of subjects, at doses exceeding the recommended nalmefene injection dose, nalmefene produced symptoms suggestive of reversal of endogenous opioids, such as nausea, chills, myalgia, dysphoria, abdominal cramps, and joint pain. These symptoms can also arise in other narcotic antagonist drugs and they are usually transient in nature and occur at a very low frequency.
Large doses of nalmefene have been used in studies. For example, one study used doses of nalmefene up to 90 mg/day for 16 weeks in patients diagnosed with pathological gambling. In a study in patients with interstitial cystitis, 20 patients received 108 mg/day of nalmefene for more than two years. Intake of a single dose of 450 mg nalmefene has been reported without changes in blood pressure, heart rate, respiration rate, or body temperature. There was no unusual pattern of adverse reactions, but the experience is limited. Management of an overdose should be observational and symptomatic.
Description
Nalmefene is a 6-methylene analogue of naltrexone. It is a pure opioid receptor antagonist and has a high affinity for the κ-opioid receptor. Nalmefene has a long duration of action and is used in oral and parenteral formulations.
Chemical properties
Off-White Solid
Originator
Nalmefene,Mallinckrodt Inc.
The Uses of Nalmefene
A structural analog of Naltrexone (N285780) with opiate antagonist activity used in pharmaceutical treatment of alcoholism. Other pharmacological applications of this compound aim to reduce food cravings, drug abuse and pulmonary disease in affected individuals. Used as an opioid-induced tranquilizer on large animals in the veterinary industry. Narcotic antagonist.
The Uses of Nalmefene
A structural labelled analog of Naltrexone (N285780) with opiate antagonist activity used in pharmaceutical treatment of alcoholism. Other pharmacological applications of this compound aim to reduce f ood cravings, drug abuse and pulmonary disease in affected individuals. Used as an opioid-induced tranquilizer on large animals in the veterinary industry. Narcotic antagonist.
The Uses of Nalmefene
opioid antagonists therapeutic for alcohol dependence
Background
Nalmefene, a 6-methylene analogue of naltrexone, is an opioid receptor antagonist. It acts as an antagonist at the mu (μ)-opioid and delta (δ)-opioid receptors and a partial agonist at the kappa (κ)-opioid receptor.
In Europe, nalmefene oral tablets are used to reduce alcohol consumption in adults with alcohol dependence. Nalmefene was approved in the United States in 1995 as an antidote for opioid overdose. Nalmefene injection is used to manage known or suspected opioid overdose. It is used for complete or partial reversal of opioid drug effects, including respiratory depression, induced by either natural or synthetic opioids. The nasal spray formulation of nalmefene was approved by the FDA in May 2023.
Indications
Nalmefene oral tablet is indicated for the reduction of alcohol consumption in adult patients with alcohol dependence who have a high drinking risk level (DRL), without physical withdrawal symptoms and who do not require immediate detoxification. Nalmefene should only be prescribed in conjunction with continuous psychosocial support focused on treatment adherence and reducing alcohol consumption. Nalmefene should be initiated only in patients who continue to have a high DRL two weeks after the initial assessment.
Nalmefene injection and nasal spray are indicated for the complete or partial reversal of opioid drug effects, including respiratory depression, induced by either natural or synthetic opioids. They are also indicated in the management of known or suspected opioid overdose. Nalmefene injection can be used for postoperative opioid overdose reversal.
Definition
ChEBI: Nalmefene is a morphinane alkaloid.
Manufacturing Process
A dry, 2-liter, 3-neck, round bottom flask fitted with two stoppers and a magnetic stirring bar was charged with potassium t-butoxide (61.1 g, 0.545 mol) and methyltriphenylphosphonium bromide (194.4 g, 0.544 mol). Freshly distilled tetrahydrofuran (450 ml) was introduced at 20°C. The resultant thick, bright yellow dispersion was stirred at 20°C for 0.5 h and further dry tetrahydrofuran (100 ml) was added. A solution of dry naltrexone (30 g, 0.088 mol) in dry tetrahydrofuran (200 ml) was then added dropwise over 40 min. Then the reaction mixture was stirred for a further 1.25 h, then cooled to 10°C, and quenched with 20% aqueous ammonium chloride solution (75 ml) followed by water (100 ml). The organic layer was separated and the aqueous layer extracted with four 100 ml portions of chloroform. Solvent was evaporated from the tetrahydrofuran layer and the combined chloroform extracts, the residues combined and brought to pH 2 by addition of 2 N hydrochloric acid. The resultant precipitate was filtered, washed with chloroform and suspended in a mixture of chloroform (500 ml) and water (250 ml). Ammonium hydroxide was added to attain a pH of 8 and the aqueous layer separated. The organic layer was dried over anhydrous sodium sulfate, filtered, and the solvent removed in vacuo. The resultant solid was dissolved in ethyl acetate (1400 ml), the solution filtered through a silica pad and the solvent evaporated. The product was recrystallized from chloroform and washed with hexane to yield pure 6-desoxy-6-methylenenaltrexone (also called nalmefene) as a white solid. Yield: 27.0 g, 88%.
Therapeutic Function
Antagonist to narcotics
Biological Functions
Nalmefene (Revex) is a long-acting injectable pure opioid antagonist recently introduced in the United States. It binds all opioid receptors and reverses the effects of opioid agonists at those receptors.The onset of action is 2 minutes after IV administration. Hepatic metabolism is slow and occurs via glucuronide conjugation to inactive metabolites. Its half-life of 11 hours is about 5 times that of naloxone. Indications include use in postoperative settings to reverse respiratory depression and in opioid overdose. Due to the long duration of action of nalmefene, however, naloxone may be preferred for treatment of overdose because it produces a shorter duration of withdrawal effects.
General Description
Nalmefene (Revex) is a pure opioid antagonist that is the6-methylene analog of naltrexone. It is available as a solutionfor IV, IM, or subcutaneous (SC) administration toreverse the effects of opioids after general anesthesia andin the treatment of overdose. It is longer acting than naloxonebut otherwise has a similar pharmacodynamic andmetabolic (3-glucuronidation) profile. Nalmefene hashigher oral bioavailability (approximately 40%) thannaloxone or naltrexone and is currently being investigatedas an oral treatment for pathological gambling and alcoholabuse.
Biochem/physiol Actions
Nonselective opioid receptor antagonist.
Pharmacokinetics
Nalmefene is an opioid antagonist with no agonist activity. It works to prevent or reverse the effects of opioids, including respiratory depression, sedation, and hypotension. Nalmefene has a longer duration of action than naloxone, another opioid antagonist used to reverse opioid overdose. In a study of brain receptor occupancy, a 1 mg dose of nalmefene blocked over 80% of brain opioid receptors within five minutes after administration.
Nalmefene has no opioid agonist activity and it is not associated with drug tolerance, physical dependence, or abuse potential.
Nalmefene is not known to produce respiratory depression, psychotomimetic effects, or pupillary constriction. No pharmacological activity was observed when nalmefene was administered in the absence of opioid agonists. However, as with all opioid antagonists, nalmefene can produce acute withdrawal symptoms in individuals with opioid dependence. These withdrawal symptoms should be managed with symptomatic and supportive treatment: the administration of large amounts of opioids to patients receiving opioid antagonists in an attempt to overcome a full blockade has resulted in adverse respiratory and circulatory reactions.
Synthesis
Nalmefene is synthesized by a
Wittig reaction of naltrexone with triphenylmethylphosphonium
bromide in DMSO under
basic catalysis of NaH.
Metabolism
Nalmefene is extensively metabolized by the liver, primarily by glucuronide conjugation mainly mediated by UGT2B7 and UGT1A3 and UGT1A8 to a lesser extent. The major metabolite is pharmacologically inactive nalmefene 3-O-glucuronide. Nalmefene is also metabolized to trace amounts of an N-dealkylated metabolite, which has minimal pharmacological activity.
Nalmefene can undergo dealkylation mediated by CYP3A4/5 to form nornalmefene. Nornalmefene can be further converted to nornalmefene 3-O-glucuronide and nornalmefene 3-sulfate, which are generally inactive metabolites.
Nalmefene can also undergo CYP3A4/5-mediated sulfation to form nalmefene 3-O-sulfate, which retains some pharmacological activity. However, nalmefene 3-O-sulfate is present in the circulation in less than 10% of that of nalmefene; thus, it is unlikely to be a major contributor to the pharmacological activity of nalmefene. The plasma concentration-time profile in some subjects suggests that nalmefene undergoes enterohepatic recycling.
Properties of Nalmefene
| Melting point: | 182-185?C |
| Boiling point: | 507.9±50.0 °C(Predicted) |
| Density | 1.38±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Dichloromethane (Slightly), DMSO (Slightly), Methanol (Sl |
| form | Solid |
| pka | pKa 7.63(H2O) (Uncertain) |
| color | White to Off-White |
| CAS DataBase Reference | 55096-26-9 |
| NIST Chemistry Reference | Nalmefene(55096-26-9) |
Safety information for Nalmefene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Nalmefene
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran

You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5