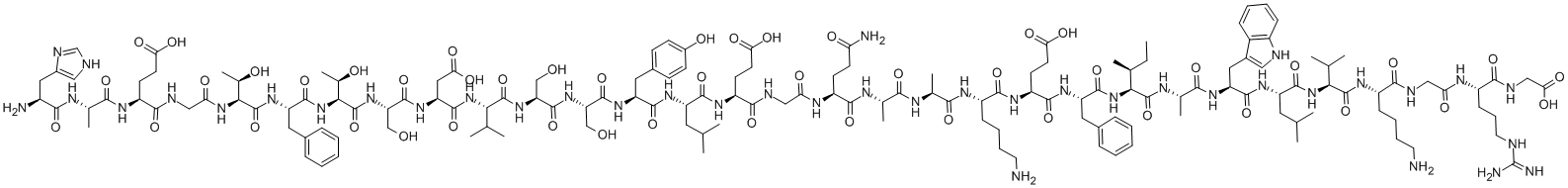
Nafarelin
- CAS NO.:76932-56-4
- Empirical Formula: C66H83N17O13
- Molecular Weight: 1322.47
- MDL number: MFCD00871253
- EINECS: 686-425-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-21 13:23:50
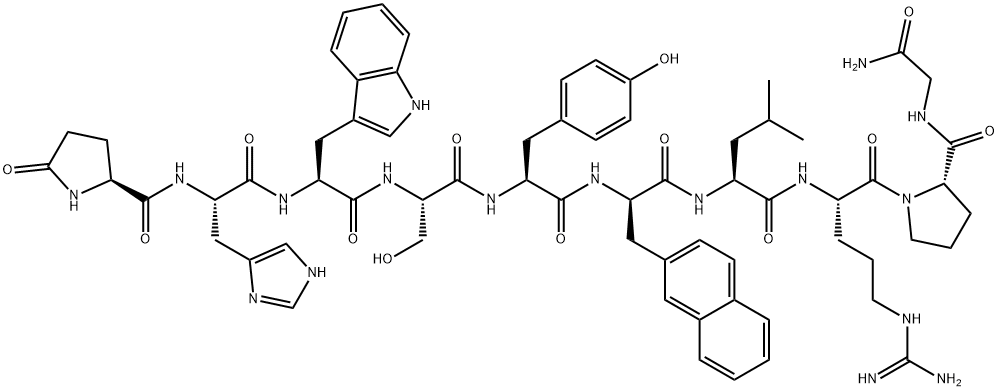
What is Nafarelin?
Absorption
Rapidly absorbed into the systemic circulation after intranasal administration. Bioavailability from a 400 μg dose averaged 2.8% (range 1.2 to 5.6%). Not absorbed after oral administration.
Toxicity
In experimental animals, a single subcutaneous administration of up to 60 times the recommended human dose (on a μg/kg basis, not adjusted for bioavailability) had no adverse effects. At present, there is no clinical evidence of adverse effects following overdosage of GnRH analogs.
Description
Nafarelin is a gonadorelin analogue. It produces an initial phase of stimulation followed by down-regulation of gonadotrophin-releasing hormone receptors, thus reducing the release of FSH and LH, leading to inhibition of androgen and oestrogen production.
Chemical properties
Nafarelin is a synthetic analogue of gonadotrophin-releasing hormone (GnRH), which is about 200 times more potent than the native hormone and is more resistant to proteolysis Chrisp and Goa (1990), Parker and Schimmer (2001). Like GnRH, it binds with high affinity to the GnRH receptor on anterior pituitary cells, where it acts as an agonist.
Originator
Nafarelin Acetate,Bachem AG
The Uses of Nafarelin
LHRH agonist.
Indications
For treatment of central precocious puberty (true precocious puberty, GnRH-dependent precocious precocity, complete isosexual precocity) in children of both sexes and for the treatment of endometriosis.
Background
Nafarelin is a potent synthetic agonist of gonadotropin-releasing hormone with 3-(2-naphthyl)-D-alanine substitution at residue 6. Nafarelin has been used in the treatments of central precocious puberty and endometriosis.
brand name
Synarel (Searle).
Therapeutic Function
Gonadotropic
Biological Functions
Acutely, Nafarelin stimulates the secretion of LH and FSH from the anterior pituitary, but prolonged, continuous exposure leads to inhibition of secretion by desensitization of the pituitary gonadotropes Vickery (1985). The initial stimulation of gonadotrophin secretion results in an increase in serum concentrations of estradiol in females and testosterone in males. These then decrease to very low concentrations equivalent to those found in menopausal women and castrated men respectively.
Pharmacokinetics
Nafarelin is a potent agonistic analog of gonadotropin-releasing hormone (GnRH). At the onset of administration, nafarelin stimulates the release of the pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), resulting in a temporary increase of gonadal steroidogenesis. Repeated dosing abolishes the stimulatory effect on the pituitary gland. Twice daily administration leads to decreased secretion of gonadal steroids by about 4 weeks; consequently, tissues and functions that depend on gonadal steroids for their maintenance become quiescent. After nafarelin therapy is discontinued, pituitary and ovarian function normalize and estradiol serum concentrations increase to pretreatment levels. Recurrences of endometriosis are frequent after cessation of any hormonal therapy, or surgery that leaves the ovaries and/or uterus intact.
Clinical Use
Nafarelin has potential wide therapeutic applications in the treatment of endometriosis, uterine leiomyoma (fibroids), malignant neoplasms, and in assisted reproduction protocols. However, its use is currently restricted to the treatment of endometriosis and in assisted reproduction protocols.
Side Effects
Nafarelin does not have the same androgenic adverse effects as danazol, but it can cause menopausal symptoms. The most commonly reported symptoms are hot flushes, vaginal dryness, altered libido and headaches. Treatment for 6 months results in a loss of bone density. The vertebral trabecular bone density falls by nearly 9% and may not return to normal after treatment. Nafarelin also has a similar effect to oestrogen deficiency on blood lipids e.g. concentrations of total cholesterol and triglycerides may increase.
Synthesis
i. 1 mmol from preparation A was placed in the reaction vessel of a 5 L Vega 296 automatic solid phase peptide synthesizer.
ii. Following amino acids were added to the Preparation A resin:
Nα-Boc-Pro 2 equiv.
Nα-Boc-Arg 2 equiv.
Nα-Boc-Leu 2 equiv.
Nα-Boc-D-NaI 1.5 equiv.
Nα-Boc-Tyr 1.5 quiv
Nα-Boc-Ser(tBu) 2 equiv.
Nα-Boc-Trp 1.75 equiv.
Nα-Boc-His(tos) 1.75 equiv.
(pyro)Glu 2.5 equiv.
iii. Crude peptide was dissolved in 2M acetic acid and converted to acetate salt by passage through a column of AG3-X4A resin.
iv. Acetate was dissolved in minimal amount of methanol.
v. It was then reprecipitated using acetone.
vi. It was then purified using HPLC to get the Nafarelin.
Metabolism
Enzymatic hydrolysis.
Properties of Nafarelin
| Density | 1.49±0.1 g/cm3(Predicted) |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly, Sonicated) |
| pka | 9.82±0.15(Predicted) |
| form | Solid |
| color | White to Off-White |
Safety information for Nafarelin
Computed Descriptors for Nafarelin
| InChIKey | RWHUEXWOYVBUCI-ITQXDASVSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Nafarelin acetate salt hydrate 97% (HPLC) CAS 76932-56-4View Details
Nafarelin acetate salt hydrate 97% (HPLC) CAS 76932-56-4View Details
76932-56-4 -
 Nafarelin acetate salt hydrate 95.00% CAS 76932-56-4View Details
Nafarelin acetate salt hydrate 95.00% CAS 76932-56-4View Details
76932-56-4 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1