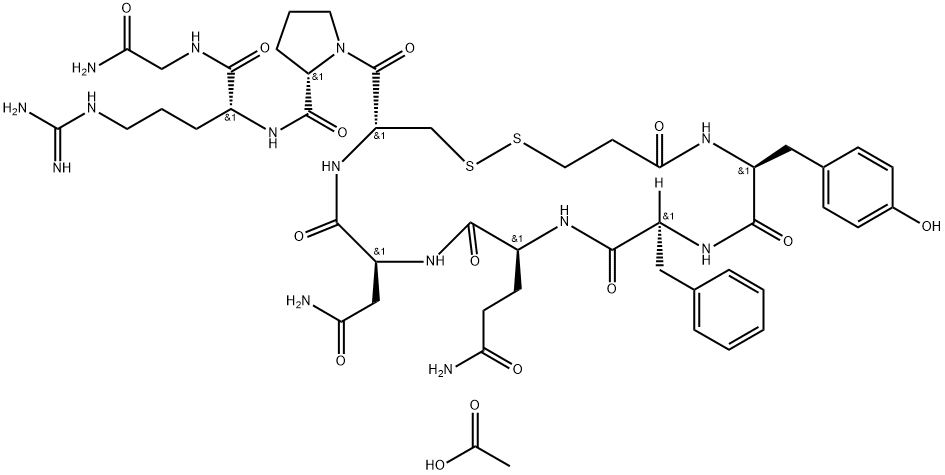
Desmopressin
- CAS NO.:16679-58-6
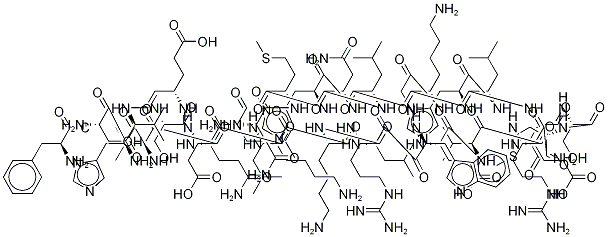
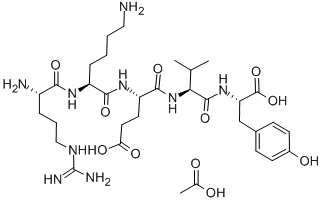
- Empirical Formula: C46H64N14O12S2
- Molecular Weight: 1069.22
- MDL number: MFCD00080163
- EINECS: 240-726-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-01 11:53:09

What is Desmopressin?
Absorption
Following nasal spray administration of 0.83 mcg and 1.66 mcg, median time to peak plasma concentrations (Tmax) was 0.25 and 0.75 hour, respectively . The peak plasma concentration was approximately 4.00 (± 3.85) pg/mL and 9.11 (± 6.90) pg/mL, respectively . The bioavailability of 1.5 mg/mL desmopressin administered by the intranasal route was between 3.3 and 4.1% .
The absolute bioavailability of orally administered desmopressin varies between 0.08% and 0.16% where the mean maximum plasma concentration is reached within 2 hours .
Toxicity
Intravenous TDLo in humans is reported to be 0.3 μg/kg/10M . Desmopressin is associated with hyponatremia in case of overdose, which may require temporary or permanent discontinuation of the therapy depending on severity. The effects of hyponatremia include seizure, altered mental status (confusion, drowsiness or continuing headache), cardiac arrhythmias and worsening edema. Other signs of overdose may include oliguria and rapid weight gain due to fluid retention . In case of overdose, reduce the dose or frequency of drug administration, or discontinue use if appropriate. Assessment of serum sodium and initiation of appropriate medical treatment is recommended.
Chemical properties
White or almost white, fluffy powder.
Originator
DAV,Ritter,Switz.,1974
The Uses of Desmopressin
Antidiuretic.
The Uses of Desmopressin
[deamino-Cys1, D-Arg8]-Vasopressin acetate salt hydrate was used to study the impact of vasopressin on pendrin abundance. E3 ubiquitin (Ub)-protein ligases (E3s) was administered in rat kidney and the changes in protein abundance of the selected E3s in response to [deamino-Cys1, D-Arg8]-Vasopressin acetate salt hydrate was examined.
Indications
Indicated for the treatment of nocturia due to nocturnal polyuria in adults who awaken at least 2 times per night to void (intranasal).
Indicated as antidiuretic replacement therapy in the management of central cranial diabetes insipidus and for management of the temporary polyuria and polydipsia following head trauma or surgery in the pituitary region (intranasal/parenteral).
Indicated for patients with hemophilia A with factor VIII coagulant activity levels greater than 5% or mild to moderate classic von Willebrand's Disease (Type I) with factor VIII levels greater than 5% during surgical procedures and postoperatively to maintain hemostasis (parenteral).
Reduce drug development failure ratesBuild, train, & validate machine-learning modelswith evidence-based and structured datasets.See how Build, train, & validate predictive machine-learning models with structured datasets.See how
Background
Desmopressin (dDAVP), a synthetic analogue of 8-arginine vasopressin (ADH), is an antidiuretic peptide drug modified by deamination of 1-cysteine and substitution of 8-L-arginine by 8-D-arginine. ADH is an endogenous pituitary hormone that has a crucial role in the control of the water content in the body. Upon release from the stimulation of increased plasma osmolarity or decreased circulating blood volume, ADH mainly acts on the cells of the distal part of the nephron and the collecting tubules in the kidney . The hormone interacts with V1, V2 or V3 receptors with differing signal cascade systems.
Desmopressin displays enhanced antidiuretic potency, fewer pressor effects due to V2-selective actions, and a prolonged half-life and duration of action compared to endogenous ADH . It has been employed clinically since 1972 and is available in various formulations including intranasal solution, intravenous solution, oral tablet and oral lyophilisate . Desmopressin is indicated for the treatment of polyuric conditions including primary nocturnal enuresis, nocturia, and diabetes insipidus. It was also newly approved for the treatment of mild classical hemophilia and von Willebrand's disease for minor surgeries. The active ingredient in most formulations is desmopressin acetate. Nocdurna, or desmopressin acetate, was approved by the FDA on June 21st, 2018 for the treatment of nocturia due to nocturnal polyuria in adults. It is available as a sublingual tablet.
What are the applications of Application
Desmopressin is a synthetic vasopressin analog
Definition
ChEBI: A synthetic analogue of vasopressin in which 3-mercaptopropionic acid replaces the cysteine residue at position 1 and D-arginine replaces the residue at position 8. An antidiuretic, it increases urine concentration and decreases urine prod ction, and is used (usually as the trihydrate of the acetic acid salt) to prevent and control excessive thirst, urination, and dehydration caused by injury, surgery, and certain medical conditions. It is also used in the diagnosis and treatment of cranial iabetes insipidus and in tests of renal function.
Manufacturing Process
β-Benzylmercaptopropionyl-L-tyrosyl-L-phenylalanyl-L-glutaminyl-Lasparaginyl-S-benzyl-L-cysteinyl-L-prolyl-NG-tosyl-D-arginyl-glycinamide (0.5 g) is reduced with sodium in liquid ammonia. The liquid ammonia is then evaporated and the residue dissolved in 5% aqueous acetic acid (800 ml). The solution is filtered to remove the undissolved portion and the filtrate is adjusted to a pH of 6.5 to 7 by addition of aqueous sodium hydroxide and it is then oxidized by known procedure, cf. Kimbrough, R.D., Jr.; Cash, W.D.; Branda, L.A.; Chan, W.Y.; and Du Vigneaud, V.; J. Biol. Chem. 238,1411 (1963). The reaction mixture is thereupon adjusted to a pH of 4 to 4.5 by addition of acetic acid. The peptide is applied to a column of a carboxylate ion exchange resin, is eluted with 50% aqueous acetic acid and isolated by lyophilization (freeze-drying). The crude product is purified by known procedure using a carrier-free high-voltage electrophoresis, cf. Zaoral, M.; Sorm, F.; Collection Czechoslov. Chem Communs, 31, 310 (1966). Yield, 100 to 200 mg of 1-deamino-8-D-argine-vasopressin.
brand name
Concentraid (Ferring Pharmaceuticals); Ddavp (Sanofi Aventis); Stimate (ZLB Behring).
Therapeutic Function
Antidiuretic
Biochem/physiol Actions
[deamino-Cys1, D-Arg8]-Vasopressin acetate salt hydrate also known as DDAVP, Desmopressin is a selective and potent vasopressor agent that stabilizes the cardiocirculatory function in normal human as well as in patients suffering from catecholamine-resistant vasodilatory shock. It also stimulate three acid-base transporters and hence increases the capability of the cell to regulate pH.
Pharmacokinetics
By mimicking the actions of endogenous ADH, desmopressin acts as a selective agonist of V2 receptors expressed in the renal collecting duct (CD) to increase water re-absorption and reduce urine production. Desmopressin has been shown to be more potent than ADH in increasing plasma levels of factor VIII activity in patients with hemophilia and von Willebrand's disease Type I . Desmopressin demonstrates markedly diminished pressor activity. Desmopressin administered intranasally has an antidiuretic effect about one-tenth that of an equivalent dose administered by injection .
Clinical Use
Diabetes insipidus
Nocturnal enuresis
Nocturia due to idiopathic nocturnal polyuria
Post-biopsy bleeding (unlicensed indication)
Pre-biopsy prophylaxis (unlicensed indication)
Metabolism
In vitro, in human liver microsome preparations, it has been shown that no significant amount of desmopressin is metabolised in the liver and thus human liver metabolism in vivo is not likely to occur .
Metabolism
Metabolic fate of desmopressin is unknown. It is not affected by liver microsomal cytochrome P450 enzymes. As a peptide, desmopressin is expected to undergo catabolism to its constituent amino acids, with subsequent recycling of the amino acid in the body pool.
storage
Store at -20°C
Properties of Desmopressin
| alpha | D25 +85.5 ± 2° (calculated for the free peptide) |
| Density | 1.56±0.1 g/cm3(Predicted) |
| RTECS | YW9000000 |
| storage temp. | Store at 0°C |
| solubility | H2O: soluble20mg/mL, clear, colorless |
| form | neat |
| pka | 9.90±0.15(Predicted) |
| Water Solubility | Soluble to 4 mg/ml in water |
Safety information for Desmopressin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. |
Computed Descriptors for Desmopressin
| InChIKey | NFLWUMRGJYTJIN-PNIOQBSNSA-N |
Desmopressin manufacturer
Shilpa Medicare Limited (SML)
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 16679-58-6 Desmopressin 99%View Details
16679-58-6 Desmopressin 99%View Details
16679-58-6 -
 16679-58-6 98%View Details
16679-58-6 98%View Details
16679-58-6 -
 Desmopressin CAS 16679-58-6View Details
Desmopressin CAS 16679-58-6View Details
16679-58-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8