N-Iodosuccinimide
Synonym(s):N-Iodosuccinimide;NIS
- CAS NO.:516-12-1
- Empirical Formula: C4H4INO2
- Molecular Weight: 224.98
- MDL number: MFCD00005512
- EINECS: 208-221-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-27 17:31:30
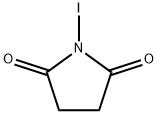
What is N-Iodosuccinimide?
Chemical properties
white-yellow to brown crystalline powder
The Uses of N-Iodosuccinimide
N-Iodosuccinimide is a iodo substituted succinimide that is used as an iodinating agent in chemical synthesis.
The Uses of N-Iodosuccinimide
Iodination of ketones and aldehydes.
Highly substituted iodobenzenes prepared via an efficient 2-step process from 1,6-diynes.
Used with TFA to chemoselectively hydrolyze thioglycosides to 1-hydroxyglycosides.
Synthesis of vinyl sulfones from olefins and benzenesulfinic acid.
The Uses of N-Iodosuccinimide
N-Iodosuccinimide is used in the preparation of vinyl sulfones from olefins and benzenesulfinic acid. It acts as a source for I+ and involved in Hunsdiecker reactions for the conversion of cinnamic acids, and propiolic acids to the corresponding alfa-halostyrenes and 1-halo-1-alkynes respectively. It is also used to hydrolyze thioglycosides to 1-hydroxyglycosides with triluoroacetic acid. It is involved in the preparation of iodobenzene from 1,6-diynes. Further, it acts as an iodinating agent in chemical synthesis.
What are the applications of Application
N-Iodosuccinimide is a reagent for the oxidimetric titration of sulfur functions, oxidations, and iodinations
Definition
ChEBI: N-iodosuccinimide is a five-membered cyclic dicarboximide compound having an iodo substituent on the nitrogen atom. It is a dicarboximide and a pyrrolidinone. It derives from a succinimide.
Preparation
To a solution of 39.2g of succinimide in 1200ml of boiling water was added 51.0g of freshly precipitated silver oxide, the mixture was filtered and the silver salt was allowed to crystallize. Filtration and washing with cold water furnished 45.0g of the silver salt of succinimide suitable for the iodination step.
The finely powdered salt (49.5g) was added in portions with stirring to a solution of 50.8g of iodine in 300ml of acetone, the temperature being maintained at 5-10°C. After decolorization (30 min.), the silver iodide was filtered, the solvent was removed under reduced pressure at room temperature and the residue was washed with ether, yielding 43g of N-iodosuccinimide with mp 189-191°C. An analytical sample (85% recovery) was obtained by dissolving in the minimum quantity of hot dioxane and precipitating with carbon tetrachloride; colorless needles, mp 200-201°C.
Ref: JACS 75, 3493 (1953)
Reactions
N-Iodosuccinimide (NIS) is an iodinating agent that is used for various electrophilic iodinations and as source for iodine in radical reactions.
N-Iodosuccinimide is the least reactive of the N-haloamides in aromatic substitution. N-Iodosuccinimide does not act as an iodinating agent in pure dimethyl sulfoxide or N-ethylacetamide. On the other hand, butyl disulfide and a number of other sulfur-containing compounds are effective catalysts at low concentration for the iodination of guanosine derivatives using N-iodosuccinimide. This catalytic effect is observed when dimethyl sulfoxide is used as the solvent,but not when N-ethylacetamide is used.
https://www.organic-chemistry.org/chemicals/oxidations/n-iodosuccinimide-nis.shtm
Synthesis Reference(s)
Journal of the American Chemical Society, 75, p. 3493, 1953 DOI: 10.1021/ja01110a055
Organic Syntheses, Coll. Vol. 5, p. 663, 1973
Tetrahedron Letters, 25, p. 233, 1984 DOI: 10.1016/S0040-4039(00)99848-4
Description
N-iodosuccinimide reacts with enol acetates derived from ketones to give α-iodoketones, and the reaction has found application in the steroid field. The iodination of the enol acetates seems to proceed by an ionic mechanism, and preliminary work indicates that N-iodosuccinimide is not capable of at least some of the radical-chain iodinations analogous to radical-chain brominations brought about by N-bromosuccinimide. N-Iodosuccinimide has also been used for the iodination of purine nucleosides and fluorenone. Iodination of 2-hydroxy-3-(β-alkyl vinyl)-1,4-naphthoquinones results in an unusual replacement of vinyl hydrogen.
Synthesis
N-iodosuccinimide could be prepared by the action of iodine on N-silver succinimide and by the action of iodine monochloride on the sodium salt of succinimide. In specific, twenty grams (0.079 mol) of iodine and 90 ml. of dried dioxane are placed in a wide-mouthed, screw-cap, brown bottle of 150–200 ml. capacity. Most of the iodine dissolves. Eighteen grams (0.087 mol) of thoroughly dried N-silver succinimide is added, and the bottle is shaken vigorously for several minutes. The mixture is occasionally shaken in the course of an hour and then is warmed in a water bath at 50° for 5 minutes. It is now filtered hot through a Büchner funnel into a 500-ml filter flask well-wrapped with black paper or aluminium foil. The silver iodide that is collected is washed with a 10-ml portion of warm dioxane. Carbon tetrachloride (200 ml) is added to the combined filtrates in the filter flask, and the solution is chilled overnight at ?8° to ?20°. N-iodosuccinimide separates as colourless crystals.
Purification Methods
Crystallise it from dioxane/CCl4. It iodinates arenes in triflic acid. [Olah et al J Org Chem 58 3194 1993, Beilstein 21/9 V 544.]
Properties of N-Iodosuccinimide
| Melting point: | 202-206 °C(lit.) |
| Boiling point: | 249.6±23.0 °C(Predicted) |
| Density | 2,245 g/cm3 |
| storage temp. | 2-8°C |
| solubility | Soluble in dioxane, tetrahydrfuran and acetonitrile. Insoluble in ether and carbon tetrachloride. |
| form | Crystalline Powder |
| appearance | White to slightly yellow/brown solid |
| pka | -2.57±0.20(Predicted) |
| color | White-yellow to brown |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,5045 |
| BRN | 113917 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 516-12-1(CAS DataBase Reference) |
Safety information for N-Iodosuccinimide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for N-Iodosuccinimide
| InChIKey | LQZMLBORDGWNPD-UHFFFAOYSA-N |
N-Iodosuccinimide manufacturer
Rivashaa Agrotech Biopharma Pvt. Ltd.
Purecha Group (Sonal Plasrub Industries Pvt Ltd)
Svaks Biotech India Pvt Ltd
ARS ChemBlocks
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 N-Iodosuccinimide 99%View Details
N-Iodosuccinimide 99%View Details -
 N-Iodosuccinimide CAS 516-12-1View Details
N-Iodosuccinimide CAS 516-12-1View Details
516-12-1 -
 N-Iodosuccinimide extrapure CAS 516-12-1View Details
N-Iodosuccinimide extrapure CAS 516-12-1View Details
516-12-1 -
 n-Iodosuccinimide CAS 516-12-1View Details
n-Iodosuccinimide CAS 516-12-1View Details
516-12-1 -
 N-Iodosuccinimide CAS 516-12-1View Details
N-Iodosuccinimide CAS 516-12-1View Details
516-12-1 -
 IODOSUCCINIMIDE For Synthesis CAS 516-12-1View Details
IODOSUCCINIMIDE For Synthesis CAS 516-12-1View Details
516-12-1 -
 N-Iodosuccinimide 98% CAS 516-12-1View Details
N-Iodosuccinimide 98% CAS 516-12-1View Details
516-12-1 -
 N-Iodosuccinimide CAS 516-12-1View Details
N-Iodosuccinimide CAS 516-12-1View Details
516-12-1