N-Ethylmorpholine
Synonym(s):4-Ethylmorpholine;N-Ethylmorpholine
- CAS NO.:100-74-3
- Empirical Formula: C6H13NO
- Molecular Weight: 115.17
- MDL number: MFCD00006177
- EINECS: 202-885-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
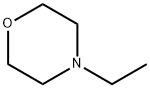
What is N-Ethylmorpholine?
Chemical properties
4-Ethylmorpholine is a colorless liquid with an ammonia-like odor.
Physical properties
Colorless, flammable liquid with an ammonia-like odor. Experimentally determined detection and recognition odor threshold concentrations were 400 μg/m3 (85 ppbv) and 1.2 mg/m3 (250 ppbv), respectively (Hellman and Small, 1974).
The Uses of N-Ethylmorpholine
N-Ethylmorpholine is a component of the buffer used in basic peptide separation through anion-exchange chromatography. Further, it acts as a catalyst in the preparation of polyurethane foam.
The Uses of N-Ethylmorpholine
Intermediate for dyestuffs, pharmaceuticals; rubber accelerators and emulsifying agents; solvent for dyes, resins, oils; catalyst in making polyurethane foams.
The Uses of N-Ethylmorpholine
Catalyst in polyurethane foam production
What are the applications of Application
4-Ethylmorpholine is a component of the buffer used in basic peptide separation via anion-exchange chromatography
Preparation
N-Ethylmorpholine is synthesized by the reaction of morpholine with bromoethane.
Synthesis Reference(s)
The Journal of Organic Chemistry, 56, p. 678, 1991 DOI: 10.1021/jo00002a035
Tetrahedron Letters, 37, p. 6749, 1996 DOI: 10.1016/S0040-4039(96)01458-X
General Description
N-Ethylmorpholine appears as a colorless liquid with a strong ammonia-like odor. Severely irritates skin, eyes, and mucous membranes. Moderately soluble in water and less dense than water. Flash point 83°F.
Air & Water Reactions
Highly flammable. Moderately soluble in water .
Reactivity Profile
N-Ethylmorpholine can react vigorously with oxidizing materials. N-Ethylmorpholine dissolves LiAlH4.
Hazard
Irritant to skin and eyes, absorbed by skin. Flammable, moderate fire risk. Toxic by skin absorption.
Health Hazard
Exposure can cause irritation of eyes, nose and throat. Contact with eyes may result in foggy vision and seeing halos around lights.
Flammability and Explosibility
Flammable
Safety Profile
oison by intravenous route. Moderately toxic by ingestion. Mildly toxic by inhalation. A skin and severe eye irritant. A very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidzing materials. To fight fire, use alcohol foam, foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of NOx.
Potential Exposure
Primary irritant (without allergic reaction). This material is used as a catalyst in polyurethane foam production. It is a solvent for dyes and resins. It is used as an intermediate in surfactant, dye, pharmaceutical, and rubber chemical manufacture
Environmental Fate
Chemical/Physical. Releases toxic nitrogen oxides when heated to decomposition (Sax and
Lewis, 1987).
At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent
concentration of 467 mg/L. The adsorbability of the carbon used was 107 mg/g carbon (Guisti et
al., 1974).
Shipping
UN2920 Corrosive liquids, flammable, n.o.s., Hazard class: 8; Labels: 8-Corrosive material, 3-Flammable liquid. UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required
Incompatibilities
May form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine,etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Corrodes some metals. Unless inhibited, violent polymerization can occur from heat, sunlight, and contact with strong oxidizers permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Attacks some plastics, rubber and coatings
Waste Disposal
Controlled incineration (oxides of nitrogen are removed from the effluent gas by scrubbers and/or thermal devices).
Properties of N-Ethylmorpholine
| Melting point: | -63 °C (lit.) |
| Boiling point: | 139 °C (lit.) |
| Density | 0.91 g/mL at 20 °C (lit.) |
| vapor pressure | 8.1 hPa (20 °C) |
| refractive index | n |
| Flash point: | 82 °F |
| storage temp. | Store below +30°C. |
| solubility | miscible |
| form | Powder |
| pka | 7.67(at 25℃) |
| color | Cream to beige to brown-grey |
| PH | 11.8 (100g/l, H2O, 20℃) |
| explosive limit | 1.9%(V) |
| Water Solubility | miscible |
| FreezingPoint | -63℃ |
| Sensitive | Air Sensitive |
| BRN | 102969 |
| Exposure limits | NIOSH REL: TWA 5 ppm (23 mg/m3), IDLH 100 ppm; OSHA PEL: TWA 20
ppm (94 mg/m3); ACGIH TLV: TWA 5 ppm (adopted). |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents. Slightly air sensitive. |
| CAS DataBase Reference | 100-74-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Morpholine, 4-ethyl-(100-74-3) |
| EPA Substance Registry System | N-Ethylmorpholine (100-74-3) |
Safety information for N-Ethylmorpholine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H311:Acute toxicity,dermal H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for N-Ethylmorpholine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 4-Ethylmorpholine 98%View Details
4-Ethylmorpholine 98%View Details -
 4-Ethylmorpholine CAS 100-74-3View Details
4-Ethylmorpholine CAS 100-74-3View Details
100-74-3 -
 n-Ethyl morpholine CAS 100-74-3View Details
n-Ethyl morpholine CAS 100-74-3View Details
100-74-3 -
 4-Ethylmorpholine CAS 100-74-3View Details
4-Ethylmorpholine CAS 100-74-3View Details
100-74-3 -
 N-Ethyl-morpholine 97% CAS 100-74-3View Details
N-Ethyl-morpholine 97% CAS 100-74-3View Details
100-74-3 -
 4-Ethylmorpholine CAS 100-74-3View Details
4-Ethylmorpholine CAS 100-74-3View Details
100-74-3 -
 4-Ethylmorpholine CAS 100-74-3View Details
4-Ethylmorpholine CAS 100-74-3View Details
100-74-3 -
 N-ETHYLMORPHOLINE For Synthesis CAS 100-74-3View Details
N-ETHYLMORPHOLINE For Synthesis CAS 100-74-3View Details
100-74-3
