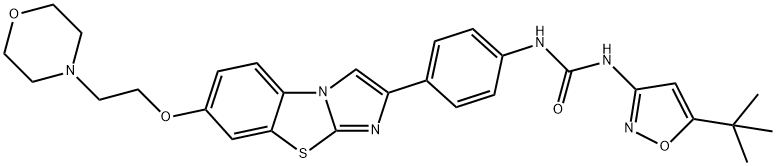
Quizartinib
- CAS NO.:950769-58-1
- Empirical Formula: C29H32N6O4S
- Molecular Weight: 560.67
- MDL number: MFCD18074524
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-03 15:10:19
What is Quizartinib ?
Absorption
The mean (SD) absolute bioavailability of quizartinib from the tablet formulation was 71% (±7%) in healthy subjects. After oral administration under fasted conditions, time to peak concentration (median Tmax) of quizartinib and AC886 measured post dose was approximately 4 hours (range 2 to 8 hours) and 5 to 6 hours (range 4 to 120 hours), respectively, in healthy subjects. Following the administration of 35.4 mg quizartinib once daily in patients with newly diagnosed acute myeloid leukemia, the Cmax and AUC0-24h were calculated to be 140 ng/mL (71%) and 2,680 ng.h/mL (85%) respectively during the induction therapy and 204 ng/mL (64%) and 3,930 ng.h/mL (78%) respectively during the consolidation therapy. For the metabolite AC886, the Cmax and AUC0-24h were estimated to be 163 ng/mL (52%) and 3,590 ng.h/mL (51%) respectively during the induction therapy and 172 ng/mL (47%) and 3,800 ng.h/mL (46%) respectively during the consolidation therapy.
Increasing the once daily dose of quizartinib to 53 mg also increases the Cmax and AUC0-24h of quizartinib to 529 ng/mL (60%) and 10,200 ng.h/mL (75%) respectively at steady state. The Cmax and AUC0-24h of the metabolite AC886 also increases to 262 ng/mL (48%) and 5,790 ng?h/mL (46%) respectively.
No clinically significant differences in the pharmacokinetics of quizartinib were observed when administered with a high-fat, high-calorie meal.
Toxicity
Based on findings from animal studies and its mechanism of action, quizartinib can cause embryo-fetal harm when administered to a pregnant woman.
There are no available data on quizartinib use in pregnant women to evaluate for a drug-associated risk. In animal reproduction studies, oral administration of quizartinib to pregnant rats during organogenesis resulted in adverse developmental outcomes including structural abnormalities and alterations to growth at maternal exposures approximately 3 times those in patients at the maximum recommended human dose (MRHD) of 53 mg/day (see Data). Advise pregnant women of the potential risk to a fetus.
Carcinogenicity studies have not been conducted with quizartinib.
Quizartinib was mutagenic in a bacterial reverse mutation (Ames) assay and not mutagenic in an in vivo transgenic rat mutation assay. Quizartinib was not genotoxic in vitro in mouse lymphoma thymidine kinase mutation and human lymphocyte chromosome aberration assays, or in an in vivo rat bone marrow micronucleus assay.
Fertility studies in animals have not been conducted with quizartinib. However, adverse findings in male and female reproductive systems were observed in repeat dose toxicity studies in rats and monkeys. Findings in female animals (rats or monkeys) included ovarian cysts, vaginal mucosal modifications, and atrophy of the uterus, ovary, and vagina, starting at exposures (AUC) approximately 0.2 times the MRHD of 53 mg/day. In male animals (rats and monkeys), findings included testicular seminiferous tubular degeneration, failure of sperm release, germ cell depletion in the testes, and oligospermia/aspermia, starting at exposures approximately 0.4 times the MRHD. After approximately one month of recovery period, all these findings except the vaginal mucosal modifications in the female rats were reversible.
Description
Quizartinib (950769-58-1) is a potent and selective inhibitor of FLT3 (Kd?= 1.6nM, IC50?= 0.56 nM MV4-11 cells).1?It is in clinical trials for treatment of Acute Myelogenous Leukemia (AML).2,3?Quizartinib priming resulted in prevention of myelosuppression in mice treated with 5-FU and Gemcitabine.4?Quizartinib showed significant reversal of ABCG2-mediated multidrug resistance (@ 3 μM)?via?antagonism of drug efflux function.5,6
The Uses of Quizartinib
AC 220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML).
Background
Quizartinib is an oral and potent fms-like tyrosine kinase 3 (FLT3) inhibitor and it is the first drug developed specifically targeting FLT3, as other agents with FLT3 inhibition activities were investigated with other targets in mind. Additionally, quizartinib also demonstrates inhibitory activity toward FLT3 with internal tandem duplication (ITD), although with a 10-fold lower affinity compared to wild-type FLT3. FLT3-ITD mutation is present in 75% of FLT3-mutated AML, leading to constitutively active FLT3 and thus poorer overall survival and higher risk of relapse. Multiple clinical trials have demonstrated quizartinib's efficacy in relapsed/refractory FLT3-ITD mutant AML. Therefore, quizartinib is proven to be a beneficial addition to the current AML treatment regimen, although serious side effects such as QT prolongation necessitates further research to optimize quizartinib's addition to AML standard of care.
Quizartinib was approved by the FDA in July 2023 and developed under the brand name VANFLYTA by Daiichi Sankyo. The FDA approval was based on positive results from the QuANTUM-First trial for FLT3-ITD positive AML, where quizartinib combined with standard cytarabine and anthracycline induction and standard cytarabine consolidation, followed by a maintenance monotherapy resulted in a 22% reduction in the risk of death.
Indications
Quizartinib is indicated in combination with standard cytarabine and anthracycline induction and cytarabine consolidation, and as maintenance monotherapy following consolidation chemotherapy, for the treatment of adult patients with newly diagnosed acute myeloid leukemia (AML) that is FLT3 internal tandem duplication (ITD)-positive as detected by an FDA-approved test.
What are the applications of Application
Quizartinib, Free Base is an Flt-3/Flk-2 inhibitor
Definition
ChEBI: A member of the class of ureas that is urea in which one of the amino groups has been substituted by a 5-tert-butyl-1,2-oxazol-3-yl group while the other has been substituted by a phenyl group substituted at the para- position by n imidazo[2,1-b][1,3]benzothiazol-2-yl group that in turn is substituted at position 7 by a 2-(morpholin-4-yl)ethoxy group.
Pharmacokinetics
Quizartinib showed antitumor activity in a mouse model of FLT3-ITD-dependent leukemia. In vitro, studies have shown that quizartinib is a predominant inhibitor of the slow delayed rectifier potassium current, IKs.
In AML patients receiving quizartinib at a dose of 90 mg/day for females and 135 mg/day for males on a 28-day schedule, the median levels of phospho-FLT3 (pFLT3) and total FLT3 (tFLT3) decreased from 3312 RLU or 5639 RLU respectively at day 1 to 1235 RLU and 142 RLU respectively at day 8. Additionally, pFLT3 levels are statistically significantly higher (p < 0.0001, Mann Whitney test) for the ITD+ subjects on day 1; however, pFLT3 levels was reduced to a similar level in patients with or without the ITD mutation.
The exposure-response analysis predicted a concentration-dependent QTcF interval median prolongation of 18 and 24 ms [upper bound of 2-sided 90% confidence interval (CI): 21 and 27 ms] at the median steady-state Cmax of quizartinib at the 26.5 mg and 53 mg dose level during maintenance therapy.
Metabolism
In vitro quizartinib is primarily metabolized via oxidation by CYP3A4/5 and AC886 is formed and metabolized by CYP3A4/5.
storage
Store at -20°C
References
1) Chao?et al.?(2009)?Identification of N-(5-tert-butyl-isoxazol-3-yl)-N’-{4-[7-(2-morpholin-4-yl-ethoxy)imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl}urea dihydrochloride (AC220), a uniquely potent, selective, and efficacious FMS-like tyrosine kinase-3 (FLT3) inhibitor; J. Med. Chem.?52?7808 2) Zarrinkar?et al.?(2009);?AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia(AML); Blood?114?2984 3) Fathi and Chen (2017);?The role of FLT3 inhibitors in the treatment of FLT3-mutated acute myeloid leukemia; Eur. J .Haematol.?98?330 4) Taylor and Langdon (2017);?Hitting the snooze button: Inducing quiescence with the FLT3 inhibitor quizartinib protects hematopoietic progenitors from chemotherapy; Mol. Cell Oncol.?4?e1378156 5) Li?et al.?(2017);?Quizartinib (AC220) reverses ABCG2-mediated multidrug resistance: In vitro and In vivo studies; Oncotarget?8?93785 6) Bhullar?et al.?(2013)?The FLT3 inhibitor quizartinib inhibits ABCG2 at pharmacologically relevant concentrations, with implications for both chemosensitization and adverse drug reactions; PLoS One?8?e71266
Properties of Quizartinib
| Density | 1.39±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to at least 25 mg/ml) |
| pka | 12.59±0.70(Predicted) |
| form | White powder. |
| color | Off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
Safety information for Quizartinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for Quizartinib
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Quizartinib 99% (HPLC) CAS 950769-58-1View Details
Quizartinib 99% (HPLC) CAS 950769-58-1View Details
950769-58-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1