Adapalene
Synonym(s):Adapalene;Differin;p75NTR;TNFRSF16;Differin, 6-(3-(1-Adamantyl)-4-methoxyphenyl)-2-naphthoic acid, CD 271, , RAR Agonist V, ADA
- CAS NO.:106685-40-9
- Empirical Formula: C28H28O3
- Molecular Weight: 412.52
- MDL number: MFCD03106112
- EINECS: 620-513-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
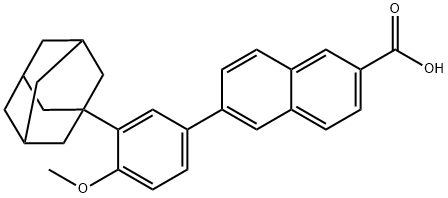
What is Adapalene?
Absorption
Adapalene is applied topically and absorbed through the skin. In one clinical study treating patients once per day with 2g of 0.3% gel applied to 2 mg/cm2 of skin, 15 patients had detectable blood plasma adapalene levels (0.1 ng/ml) resulting in a mean Cmax of 0.553 ± 0.466 ng/ml and a mean AUC of 8.37 ± 8.46 ng*h/ml on day 10.
Toxicity
Toxicity information regarding adapalene is not readily available. Patients experiencing an overdose are at an increased risk of severe adverse effects such as redness, scaling, and skin discomfort. Symptomatic and supportive measures are recommended.
Adapalene has an acute oral LD50 in S-D rats and CD-1 mice of over 5000 mg/kg. The LD50 of 0.3% applied topically to Credo OF1 mice is over 10 ml/kg (30 mg/kg). No systemic or local toxicity was observed in rats treated topically with 6 mg/kg/day of 0.3% adapalene.
Description
Adapalene is a third-generation topical retinoid used for the treatment of acne vulgaris. It is an agonist of retinoic acid receptors (RARs; Kds = 1,100, 34, and 130 nM for RARα, RARβ, and RARγ, respectively). It inhibits growth and differentiation of sebocytes in a concentration-dependent manner in primary rat preputial cell culture. Adapalene (10 μM) completely inhibits the activity of soybean 15-lipoxygenase (15-LOX) in an enzyme assay and inhibits the 5- and 15-LOX pathways in human blood polymorphonuclear leukocytes (PMNs). Adapalene (100 μM) also induces apoptosis and inhibits proliferation of CC-531, HT-29, and LoVo colon cancer cells and reduces tumor growth in a DLD-1 colon cancer nude mouse xenograft model in a dose-dependent manner.
Chemical properties
White Crystalline Solid
The Uses of Adapalene
Adapalene has been used as a retinoic acid analog and apoptosis inducer to study its effects on inhibiting transcription of hepatitis B virus in covalently closed circular DNA (cccDNA). It has also been used as a component to culture mixed lymphocyte reactions (MLR) to study its effects on isolated macrophages.
Indications
Adapalene is indicated for the topical treatment of acne vulgaris in patients aged 12 and over. It is also indicated for acne vulgaris in combination with benzoyl peroxide and in a triple combination therapy with benzoyl peroxide and clindamycin.
Definition
ChEBI: A naphthoic acid that is CD437 in which the phenolic hydroxy group has been converted to its methyl ether.
Biochem/physiol Actions
Retinoic acid analogue that is a RARβ and RARγ agonist (AC50 values are 2.2, 9.3, 22 and > 1000 nM for RARβ, RARγ, RARα and RXRα receptors respectively). Inhibits proliferation and induces apoptosis in colorectal cancer cells in vitro. Displays comedolytic activity. Its unique pharmacological properties make it superior to other retinoids for the treatment of acne.
Pharmacokinetics
Adapalene is anticomedogenic, preventing the formation of new comedones and inflammatory lesions, and also acts to reduce inflammation by modulating the innate immune response. Like other retinoid compounds, adapalene is chemically stable but photosensitive; use with sunscreen is recommended. Minor skin irritations, including erythema, scaling, dryness, and stinging/burning, have been reported.
Metabolism
Extensive information regarding adapalene metabolism in humans is unavailable, although it is known to accumulate in the liver and GI-tract. In human, mouse, rat, rabbit, and dog cultured hepatocytes, metabolism appears to affect the methoxybenzene moiety but remains incompletely characterized. The major products of metabolism are glucuronides. Approximately 25% of the drug is metabolized; the rest is excreted as parent drug.
Structure and conformation
Retinoid-like compound that binds to retinoic acid nuclear receptors, but not to cytoplasmic receptor proteins.
References
1) Michel et al. (1998), Pharmacology of Adapalene; Br. J. Dermatol., 139 Suppl. 52 3 2) Ocker et al. (2003), The synthetic retinoid adapalene inhibits proliferation and induces apoptosis in colorectal cancer cells on vitro; Int. J. Cancer, 107 453 3) Milikan et al. (2000), Adapalene: an update on newer comparative studies between the various retinoids; Int. J. Dermatol., 39 784 4) Bernard et al. (1993), Adapalene, a new chemical entity with retinoid activity; Skin Pharmacol., 6 61
Properties of Adapalene
| Melting point: | 319-3220C |
| Boiling point: | 606.3±55.0 °C(Predicted) |
| Density | 1.233±0.06 g/cm3(Predicted) |
| RTECS | QJ1987000 |
| storage temp. | 2-8°C |
| solubility | DMSO: >10mg/mL |
| pka | 4.2(at 25℃) |
| form | White solid |
| color | white to off-white |
| Merck | 14,150 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or DMF may be stored at -20°C for up to 3 months |
| CAS DataBase Reference | 106685-40-9(CAS DataBase Reference) |
Safety information for Adapalene
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Adapalene
| InChIKey | LZCDAPDGXCYOEH-UHFFFAOYSA-N |
| SMILES | C1=C2C(C=C(C3=CC=C(OC)C(C45CC6CC(CC(C6)C4)C5)=C3)C=C2)=CC=C1C(O)=O |
Adapalene manufacturer
Omicron Pharmatech Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Adapalene 106685-40-9 98%View Details
Adapalene 106685-40-9 98%View Details
106685-40-9 -
 106685-40-9 98%View Details
106685-40-9 98%View Details
106685-40-9 -
 Adapalene 99%View Details
Adapalene 99%View Details
106685-40-9 -
 Adapalene 99%View Details
Adapalene 99%View Details -
 Adapalene CAS 106685-40-9View Details
Adapalene CAS 106685-40-9View Details
106685-40-9 -
 Adapalene >98% (HPLC) CAS 106685-40-9View Details
Adapalene >98% (HPLC) CAS 106685-40-9View Details
106685-40-9 -
 Adapalene 95.00% CAS 106685-40-9View Details
Adapalene 95.00% CAS 106685-40-9View Details
106685-40-9 -
 99.99% Adapalene API powder, 106685-40-9View Details
99.99% Adapalene API powder, 106685-40-9View Details
106685-40-9
