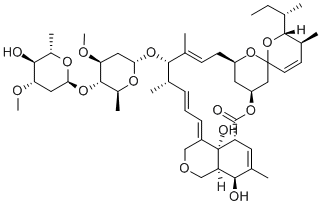
Moxidectin
Synonym(s):Moxidectin
- CAS NO.:113507-06-5
- Empirical Formula: C37 H53 N O8
- Molecular Weight: 639.82
- MDL number: MFCD00866560
- EINECS: 635-129-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-19 17:50:00
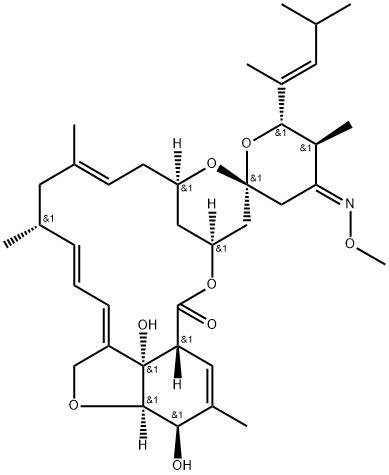
What is Moxidectin?
Absorption
The penetration of moxidectin in the parasite is not restricted as this compound is a very poor substrate of p-glycoprotein, which is vital for the reduction of the uptake of lipophilic compounds from the GI tract and for the increase in biliary, intestinal and renal secretion. After oral administration of moxidectin, the plasma maximal concentration of 70.4 mg/kg was reached after 0.37 day with a reported AUC of 363.6 mcg/day/ml. It is also important to mention that oral bioavailability is enhanced with the co-administration with lipids.
Toxicity
Moxidectin is reported to be safe as it is reported to be a poor substrate of P-glycoprotein. The reported LD50in mice are in the range of 70-131 micromol/kg. Carcinogenicity studies have not been performed. Moxidectin was shown to present no effects in genotoxicity, mutagenicity and fertility. The reports of overdose there are related to the presence of transient and self-limiting neurological signs including the presence of convulsions.
Description
Moxidectin is a macrocyclic lactone endectocide and a derivative of nemadectin. It reduces fecal nematode egg counts by 98.4 and 99.8% in naturally infected calves when administered subcutaneously at doses of 0.2 and 0.3 mg/kg, respectively. Moxidectin (0.2 and 0.3 mg/kg) reduces the worm burden of O. ostertagi and T. axei in the abomasum, and Cooperia species and N. helvetianus in the small intestine, of naturally infected calves. It potentiates GABA-gated currents in Xenopus oocytes expressing rat α1β2γ2 subunit-containing GABAA receptors with an EC50 value of 11.8 nM. Formulations containing moxidectin have been used in the treatment of onchocerciasis, as well as in the prevention and treatment of parasitic infections in veterinary medicine.
Description
The macrocyclic lactone moxidectin is an anthelmintic drug, which means that it is used to control or prevent parasitic worms (helminths). Its principal use is veterinary, in animals ranging from dogs and cats to cattle and horses. Parasites susceptible to moxidectin include Strongylus vulgaris (“blood worm”) in horses and Ostertagia ostertagi (“brown stomach worm”) in cattle.
Moxidectin, obtained from Streptomyces bacteria, was developed in the 1980s by the now-defunct American Cyanamid Co. Today, it is marketed worldwide under several trade names by major pharma companies such as Bayer Animal Health, Pfizer Animal Health, and Zoetis.
This year, on the basis of only two double-blind studies, the US Food and Drug Administration approved the use of moxidectin on humans. The target disease is river blindness (onchocerciasis), which occurs mainly in sub-Saharan Africa. The disease is caused by the parasitic worm Onchocerca volvulus, which is disseminated by Simulium species of black flies.
Moxidectin was registered by the nonprofit Medicines Development for Global Health (MDGH) with guidance from a World Health Organization initiative. MDGH is the first nonprofit to register a tropical medicine drug through FDA’s priority review voucher program.
The Uses of Moxidectin
Moxidectin is a macrocyclic lactone and semisynthetic derivative of nemadectin. Moxidectin is a parasiticide used for the prevention and control of heartworm and intestinal worms. Moxidectin is is fou nd in veterinary medicine used to treat animals such as dogs, cats, horses, cattle and sheep.
The Uses of Moxidectin
anthelmintic, antiparasitic
The Uses of Moxidectin
Moxidectin is a semi-synthetic milbemycin derived from nemadectin by selective oxidation followed by methyloximation. Moxidectin was patented in 1991 as an anthelmintic for internal parasite control. The presence of the methyloxime affords moxidectin a greater hydrophobicity and longer biological half-life compared to nemadectin. Moxidectin binds selectively to parasite glutamate-gated chloride ion channels and disrupts neurotransmission leading to paralysis and death of the parasite.
Indications
Moxidectin is indicated for the treatment of river blindness, also called onchocerciasis, in patients aged 12 years and older. River blindness is caused by a parasitic worm Onchocerca volvulus and it is manifested as severe itching, disfiguring skin conditions and visual impairment caused by the worm's larvae.
The transmission of Onchocerca volvulus is performed person to person by black flies that breed in fast-flowing rivers in sub-Saharan Africa, Yemen and South and Central America. The larvae released by the adult parasite invade skin and eyes where they can produce the severe disease manifestations.
Background
Moxidectin is a potent, broad-spectrum endectocide (antiparasitic that is active against endo- and ecto-parasites) with activity against nematodes, insects, and acari. It was first used in cattle followed by an approved use in general animals. It is a semi-synthetic methoxine derivative of nemadectin which is a 16-member pentacyclic lactone of the milbemycin class. Moxidectin differs by the absence of a disaccharide moiety on carbon 13, a substituted olefinic side chain at carbon 25 and a unique methoxime moiety at carbon 23. Due to these modifications, moxidectin is classified as a second generation macrocyclic lactone. Moxidectin was developed by Medicines Development for Global Health and FDA approved in June 13, 2018.
Definition
ChEBI: Moxidectin is a milbemycin.
Pharmacokinetics
Moxidectin has been reported to be highly effective against Onchocerca volvulus when compared to ivermectin. When moxidectin was administered in infected individuals, the microfilarial load in the skin was lower even when compared to the current therapy, ivermectin. The levels of microfilarial got reduced to an undetectable level while being safe to be used in mass drug administration.
Veterinary Drugs and Treatments
In dogs and cats, moxidectin with lufenuron is indicated as a once
a month topical preventative for the prevention of heartworm, flea
adulticide, ear mites (cats) and treatment for hookworms, roundworms,
and whipworms (dogs). It has also been successfully used as
a treatment for generalized demodicosis.
In cattle, moxidectin is indicated for the treatment and control
of the following internal [adult and fourth stage larvae (L4)]
and external parasites: Gastrointestinal roundworms: Ostertagia
ostertagi (adult and L4, including inhibited larvae), Haemonchus
placei (adult), Trichostrongylus axei (adult and L4), Trichostrongylus
colubriformis (adult), Cooperia oncophora (adult), Cooperia punctata
(adult), Bunostomum phlebotomum (adult), Oesophagostomum
radiatum (adult), Nematodirus helvetianus (adult); Lungworm:
Dictyocaulus viviparus (adult and L4); Cattle Grubs: Hypoderma
bovis, Hypoderma lineatum Mites: Chorioptes bovis, Psoroptes
ovis (Psoroptes communis var. bovis); Lice: Linognathus vituli,
Haematopinus eurysternus, Solenopotes capillatus, Damalinia bovis;
Horn flies: Haematobia irritans. To control infections and to protect
from reinfection from Ostertagia ostertagi for 28 days after treatment
and from Dictyocaulus viviparus for 42 days after treatment.
In sheep, oral moxidectin is indicated for the control of
Haemonchus contortus (adult and L4), Teladosrsagia circumcincta
& trifurcata (adult and L4), Trichostrongylus colubriformis, axei, &
vitrinius (adult & L4), Cooperia curticei & oncophora (adult and L4),
Oesophagostomum columbianum & venolosum (adult & L4), and
Nematodirus battus, filicollis, & spathiger (adult & L4).
In horses and ponies, moxidectin is indicated for the treatment
and control of the following stages of gastrointestinal parasites:
Large strongyles: Strongylus vulgaris (adults and L4L5 arterial
stages); Strongylus edentatus (adults and tissue stages);
Triodontophorus brevicauda (adults); Triodontophorus serratus
(adults); Small strongyles (adults and larvae): Cyathostomum spp.
(adults); Cylicocyclus spp. (adults); Cylicostephanus spp. (adults);
Gyalocephalus capitatus (adults); undifferentiated lumenal larvae;
Encysted cyathostomes: late L3 and L4 mucosal cyathostome larvae;
Ascarids: Parascaris equorum (adults and L4 larval stages); Pin
worms: Oxyuris equi (adults and L4 larval stages); Hair worms:
Trichostrongylus axei (adults); Large-mouth stomach worms:
Habronema muscae (adults); Horse stomach bots: Gasterophilus intestinalis
(2nd and 3rd instars). When combined with praziquantel,
additional coverage against Anoplocephala spp. occurs.
Metabolism
Reports have registered enzymatic modification in humans and in nematodes. In the case of moxidectin, there has been registered C29-30- and C14-mono-hydroxymethyl derivatives mainly by the cytochrome CYP3A and CYP2B. The metabolism of moxidectin is considered to contribute to a small extent to the elimination. Some other metabolites formed are O-demethyl-dihydroxy metabolites. The metabolism of the of moxidectin is not major as the major residue in fat, liver, kidney and muscle is the unchanged moxidectin.
Properties of Moxidectin
| Melting point: | 132 °C |
| Boiling point: | 790.0±70.0 °C(Predicted) |
| Density | 1.23±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | ≥128 mg/mL in EtOH; ≥129.4 mg/mL in DMSO; ≥3.27 mg/mL in H2O with gentle warming and ultrasonic |
| appearance | white to pale yellow crystals or power |
| pka | 12.46±0.70(Predicted) |
| form | neat |
| form | Solid |
| color | White to Almost white |
| Stability: | Light Sensitive |
Safety information for Moxidectin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. |
Computed Descriptors for Moxidectin
| InChIKey | YZBLFMPOMVTDJY-LSGXYNIPSA-N |
Moxidectin manufacturer
Diligent Life Care
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Moxidectin 96% (HPLC) CAS 113507-06-5View Details
Moxidectin 96% (HPLC) CAS 113507-06-5View Details
113507-06-5 -
 Moxidectin 98% CAS 113507-06-5View Details
Moxidectin 98% CAS 113507-06-5View Details
113507-06-5 -
 Moxidectin CAS 113507-06-5View Details
Moxidectin CAS 113507-06-5View Details
113507-06-5 -
 Moxidectin CAS 113507-06-5View Details
Moxidectin CAS 113507-06-5View Details
113507-06-5 -
 Moxidectin CAS 113507-06-5View Details
Moxidectin CAS 113507-06-5View Details
113507-06-5 -
 Moxidectin CAS 113507-06-5View Details
Moxidectin CAS 113507-06-5View Details
113507-06-5 -
 113507-06-5 Moxidectin 98%View Details
113507-06-5 Moxidectin 98%View Details
113507-06-5 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1