Isoconazole
- CAS NO.:27523-40-6
- Empirical Formula: C18H14Cl4N2O
- Molecular Weight: 416.13
- EINECS: 248-508-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
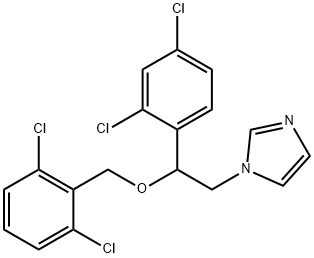
What is Isoconazole?
Chemical properties
White or almost white powder.
Originator
Fazol,Fournier,France,1979
The Uses of Isoconazole
Antibacterial; antifungal.
Background
Isoconazole is an azole antifungal drug that has similar effectiveness to clotrimazole in the treatment of foot and vaginal infections.
Definition
ChEBI: 1-{2-[(2,6-dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl}imidazole is a member of the class of imidazoles that carries a 2-[(2,6-dichlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl group at position 1. It is a dichlorobenzene, an ether and a member of imidazoles.
Indications
Topical treatment of mycoses of the skin induced or sustained by fungi such as dermatophytes and yeasts. In addition, isoconazole is indicated for therapy of vulvovaginal mycoses causes by Candida species and Candida (Torulopsis) glabrata.
Manufacturing Process
To a stirred and refluxing solution of 40 parts of benzene and 35 parts of dimethylformamide (both solvents previously dried azeotropically) are added successively 1.6 parts of sodium hydride and 7.7 parts of α-(2,4- dichlorophenyl)imidazole-1-ethanol, (cooling on ice is necessary). After the addition is complete, stirring and refluxing is continued for 30 minutes. Then there are added 7.8 parts of 2.6-dichlorobenzyl chloride and the whole is stirred at reflux for another 3 hours. The reaction mixture is poured onto water and the product 1-[2,4-dichloro-b-(2,6-dichlorobenzyloxy)phenethyl] imidazole, is extracted with benzene. The extract is washed twice with water, dried, filtered and evaporated in vacuo. The base residue is dissolved in a mixture of acetone and diisopropyl ether and to this solution is added an excess of concentrated nitric acid solution. The precipitated nitrate salt is filtered off and recrystallized from a mixture of methanol and diisopropyl ether, yielding 1-[2,4-dichloro-b-(2,6-dichlorobenzyloxy)phenethyl]imidazole nitrate; melting point 179°C.
Therapeutic Function
Antibacterial, Antifungal
Antimicrobial activity
Broad-spectrum azole antimycotic with activity against almost all species of pathogenic fungi.
Pharmacokinetics
After topical application, isoconazole remains practically unabsorbed. Application in an ethanol/propyleneglycol vehicle increases penetration into the stratum corneum of the skin significantly.
Side Effects
Local irritations such as itching and burning sensations and allergic reactions may occur in rare cases and are mainly due to the galenic formulation.
Metabolism
Not Available
Solubility in organics
Isoconazole is soluble in methanol, acetic acid, and PEG 400; less soluble in ethanol, acetone, chloroform, and butanol; and barely soluble in water, diethyl ether, cyclohexane, and hexane.
Properties of Isoconazole
| Boiling point: | 555.1±50.0 °C(Predicted) |
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, very soluble in methanol, freely soluble in ethanol (96 per cent). |
| form | neat |
| pka | 6?+-.0.12(Predicted) |
| CAS DataBase Reference | 27523-40-6 |
Safety information for Isoconazole
Computed Descriptors for Isoconazole
Isoconazole manufacturer
Clickchem Research LLP
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 27523-40-6 98%View Details
27523-40-6 98%View Details
27523-40-6 -
 Isoconazole 98%View Details
Isoconazole 98%View Details
27523-40-6 -
 Isoconazole CAS 27523-40-6View Details
Isoconazole CAS 27523-40-6View Details
27523-40-6 -
 27523-40-6 MICONAZOLE EP IMPURITY D/2,6-ISOMER 90 % AboveView Details
27523-40-6 MICONAZOLE EP IMPURITY D/2,6-ISOMER 90 % AboveView Details
27523-40-6 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
